Abstract
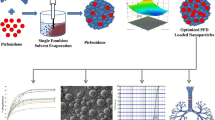
This study evaluated the feasibility of ciprofloxacin nanocrystals dry powder inhalations (DPIs) co-delivered with N-acetylcysteine (NAC) to improve the drug permeability and pulmonary bioavailability. The NAC could be reducing the viscosity of the sputum and causing it to liquefy, by breaks DNA fibers in sputum and purulent sputum. The ciprofloxacin nanocrystals, stabilized by 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine grafted N-[methoxy (polyethylene glycols)-2000] (DSPE-mPEG2000), was prepared by anti-solvent precipitation technology. The powder contained ciprofloxacin nanocrystals and N-acetyl-cysteine was obtained as a result of freeze-drying and spray-drying, respectively. The powder was coupled with inhaled lactose and the DPI of ciprofloxacin nanocrystals/NAC co-delivery system was obtained. The in vitro characteristics, including particle size and distribution, morphology, crystalline state, stability, flowability, aerosol performance, and in vivo image were evaluated. The spray-dried DPI of ciprofloxacin nanocrystals/NAC co-delivery system exhibited stratified in vitro and in vivo aerosol performance. This study demonstrated that spray-dried DPI system with a synergistic effect and meanwhile a better flowability is desirable for enhanced pulmonary drug delivery without severe lung injury.








Similar content being viewed by others
Abbreviations
- DPIs:
-
Dry powder inhalations
- DPI:
-
Dry powder inhaler
- NAC:
-
N-acetyl-cysteine
- DSPE-mPEG2000:
-
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine grafted N-[methoxy (polyethylene glycols)-2000]
- MIC:
-
Minimum inhibitory concentration
- MDI:
-
Metered-dose inhaler
- CPFX:
-
Ciprofloxacin
- NCFB:
-
Non-cystic fibrosis bronchiectasis
- mPEG2000:
-
Methoxy-polyethylene glycols-2000
- DSPE:
-
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine
- PVP K-30:
-
Polyvinylpyrrolidone K-30
- CDI:
-
N,N′-Carbonyldiimidazole
- DMAP:
-
4-Dimethylaminopyridine
- DiR:
-
1,1-Dioctadecyl-3,3,3,3-tetramethylindotricarbocyaine iodide
- FTIR:
-
Fourier transform infrared spectroscopy
- 1H NMR:
-
1H-nuclear magnetic resonance
- DLS:
-
Dynamic light scattering
- PDI:
-
Polydispersity index
- TEM:
-
Transmission electron microscope
- CI:
-
Carr’s index
- SEM:
-
Scanning electron microscope
- XRPD:
-
X-ray powder diffractometer
- DSC:
-
Differential scanning calorimetry
- TGA:
-
Thermogravimetric analysis
- TSI:
-
Twin stage impinger
- ED:
-
Emitted dose
- RD:
-
Recovered dose
- FPF:
-
Fine particle fraction
References
Abdelaziz HM, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM, Kamel NM, Kabary DM, Freag MS, Samaha MW, Mortada SM, Elkhodairy KA, Fang J-Y, Elzoghby AO (2018) Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release 269:374–392. https://doi.org/10.1016/j.jconrel.2017.11.036
Alhajj N, O’Reilly NJ, Cathcart H (2021) Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol 384:313–331. https://doi.org/10.1016/j.powtec.2021.02.031
Almansour, K., Alfagih, I.M., Ali, R., Elsayed, M.M.A. (2020) Inhalable microparticles containing terbinafine for management of pulmonary fungal infections: spray drying process engineering using lactose vs. mannitol as excipients. Journal of Drug Delivery Science and Technology 60:101991. https://doi.org/10.1016/j.jddst.2020.101991
Alothman GA, Alsaadi MM, Ho BL, Ho SL, Dupuis A, Corey M, Coates AL (2002) Evaluation of bronchial constriction in children with cystic fibrosis after inhaling two different preparations of tobramycin. Chest 122:930–934. https://doi.org/10.1378/chest.122.3.930
Babu NJ, Nangia A (2011) Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst Growth Des 11:2662–2679. https://doi.org/10.1021/cg200492w
Bosquillon C, Préat V, Vanbever R (2004) Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats. J Control Release 96:233–244. https://doi.org/10.1016/j.jconrel.2004.01.027
Carpenter JF, Crowe JH (1988) The mechanism of cryoprotection of proteins by solutes. Cryobiology 25:244–255. https://doi.org/10.1016/0011-2240(88)90032-6
Carpenter JF, Crowe JH (1989) An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 28:3916–3922. https://doi.org/10.1021/bi00435a044
Chandel A, Goyal AK, Ghosh G, Rath G (2019) Recent advances in aerosolised drug delivery. Biomed Pharmacother 112:108601. https://doi.org/10.1016/j.biopha.2019.108601
Chew NYK, Shekunov BY, Tong HHY, Chow AHL, Savage C, Wu J, Chan H-K (2005) Effect of amino acids on the dispersion of disodium cromoglycate powders. J Pharm Sci 94:2289–2300. https://doi.org/10.1002/jps.20426
d’Angelo I, Conte C, La Rotonda MI, Miro A, Quaglia F, Ungaro F (2014) Improving the efficacy of inhaled drugs in cystic fibrosis: challenges and emerging drug delivery strategies. Adv Drug Deliv Rev 75:92–111. https://doi.org/10.1016/j.addr.2014.05.008
de Boer AH, Thalberg K (2021) Chapter 5 - Dry powder inhalers (DPIs). In: Kassinos S, Bäckman P, Conway J, Hickey AJ (eds) Inhaled medicines. Academic Press, pp 99–146
Dong L, Sun L, Li W, Jiang Y, Zhan Y, Yu L, Chen Y, Hong G (2021) Degradable and Excretable ultrasmall transition metal selenide nanodots for high-performance computed tomography bioimaging-guided photonic tumor nanomedicine in NIR-II Biowindow. Adv Func Mater 31:2008591. https://doi.org/10.1002/adfm.202008591
Guan W, Ma Y, Ding S, Liu Y, Song Z, Liu X, Tang L, Wang Y (2022) The technology for improving stability of nanosuspensions in drug delivery. J Nanopart Res 24:14. https://doi.org/10.1007/s11051-022-05403-9
Ho D-K, Nichols BLB, Edgar KJ, Murgia X, Loretz B, Lehr C-M (2019) Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur J Pharm Biopharm 144:110–124. https://doi.org/10.1016/j.ejpb.2019.09.002
Hong Z, Chen D, Zhou W, Zeng Y, Wang Q (2018) Effect of novel combinatorial nanosuspension of catechin and ciprofloxacin on E. coli induced prostatitis rat model. Nanosci Nanotechnol Lett 10:1606–1612. https://doi.org/10.1166/nnl.2018.2809
Hudson R, Olson Blair B (2011) Inhaled antibiotics for Gram-negative respiratory infections. Future Med Chem 3:1663–1677. https://doi.org/10.4155/fmc.11.114
Jong WHD, Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomed 3:133–149. https://doi.org/10.2147/IJN.S596
Khatib I, Khanal D, Ruan J, Cipolla D, Dayton F, Blanchard JD, Chan H-K (2019) Ciprofloxacin nanocrystals liposomal powders for controlled drug release via inhalation. Int J Pharm 566:641–651. https://doi.org/10.1016/j.ijpharm.2019.05.068
Khatib I, Tang P, Ruan J, Cipolla D, Dayton F, Blanchard JD, Chan H-K (2020) Formation of ciprofloxacin nanocrystals within liposomes by spray drying for controlled release via inhalation. Int J Pharm 578:119045. https://doi.org/10.1016/j.ijpharm.2020.119045
Lababidi N, Montefusco-Pereira CV, de Souza Carvalho-Wodarz C, Lehr C-M, Schneider M (2020) Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur J Pharm Biopharm 157:200–210. https://doi.org/10.1016/j.ejpb.2020.10.010
Liao Q, Yip L, Chow MYT, Chow SF, Chan HK, Kwok PCL, Lam JKW (2019) Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int J Pharm 560:144–154. https://doi.org/10.1016/j.ijpharm.2019.01.057
Liu Y, Wang Y, Zhao J (2019) Design, optimization and in vitro-in vivo evaluation of smart nanocaged carrier delivery of multifunctional PEG-chitosan stabilized silybin nanocrystals. Int J Biol Macromol 124:667–680. https://doi.org/10.1016/j.ijbiomac.2018.11.258
Ma Y, Gao J, Jia W, Liu Y, Zhang L, Yang Q, Guo J, Zhao J, Yan B, Wang Y (2020a) A Comparison of spray-drying and freeze-drying for the production of stable silybin nanosuspensions. J Nanosci Nanotechnol 20:3598–3603. https://doi.org/10.1166/jnn.2020.17407
Ma Y, Wang Y, Wang X, Guo J, Yan B, Guan W (2020b) Development and solidification of multifunction stabilizers formulated self-assembled core-shell Deacetyl mycoepoxydience nanosuspensions. J Mol Liq 312:113480. https://doi.org/10.1016/j.molliq.2020.113480
Marques, M.R.C., Loebenberg, R., Almukainzi, M. (2011) Simulated biological fluids with possible application in dissolution testing. Dissolution Technologies 18:15–28. https://doi.org/10.14227/DT180311P15
McShane PJ, Weers JG, Tarara TE, Haynes A, Durbha P, Miller DP, Mundry T, Operschall E, Elborn JS (2018) Ciprofloxacin dry powder for inhalation (ciprofloxacin DPI): technical design and features of an efficient drug–device combination. Pulm Pharmacol Ther 50:72–79. https://doi.org/10.1016/j.pupt.2018.03.005
Merlos R, Wauthoz N, Levet V, Belhassan L, Sebti T, Vanderbist F, Amighi K (2017) Optimization and scaling-up of ITZ-based dry powders for inhalation. Journal of Drug Delivery Science and Technology 37:147–157. https://doi.org/10.1016/j.jddst.2016.12.009
Millan DS, Ballard SA, Chunn S, Dybowski JA, Fulton CK, Glossop PA, Guillabert E, Hewson CA, Jones RM, Lamb DJ, Napier CM, Payne-Cook TA, Renery ER, Selby MD, Tutt MF, Yeadon M (2011) Design and synthesis of long acting inhaled corticosteroids for the treatment of asthma. Bioorg Med Chem Lett 21:5826–5830. https://doi.org/10.1016/j.bmcl.2011.07.106
Njälsson R, Norgren S (2005) Physiological and pathological aspects of GSH metabolism. Acta Paediatr 94:132–137. https://doi.org/10.1080/08035250410025285
Osman R, Kan PL, Awad G, Mortada N, El-Shamy A-E, Alpar O (2013a) Spray dried inhalable ciprofloxacin powder with improved aerosolisation and antimicrobial activity. Int J Pharm 449:44–58. https://doi.org/10.1016/j.ijpharm.2013.04.009
Osman R, Kan PL, Awad G, Mortada N, El-Shamy AE, Alpar O (2013b) Spray dried inhalable ciprofloxacin powder with improved aerosolisation and antimicrobial activity. Int J Pharm 449:44–58. https://doi.org/10.1016/j.ijpharm.2013.04.009
Otroj M, Taymouri S, Varshosaz J, Mirian M (2020) Preparation and characterization of dry powder containing sunitinib loaded PHBV nanoparticles for enhanced pulmonary delivery. Journal of Drug Delivery Science and Technology 56:101570. https://doi.org/10.1016/j.jddst.2020.101570
Rubin BK, Williams RW (2014) Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. Respiration 88:177–184. https://doi.org/10.1159/000366000
Santos J, Calero N, Trujillo-Cayado LA, Garcia MC, Muñoz J (2017) Assessing differences between Ostwald ripening and coalescence by rheology, laser diffraction and multiple light scattering. Colloids Surf, B 159:405–411. https://doi.org/10.1016/j.colsurfb.2017.08.015
Ståhl K, Claesson M, Lilliehorn P, Lindén H, Bäckström K (2002) The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int J Pharm 233:227–237. https://doi.org/10.1016/S0378-5173(01)00945-0
Steckel H, Brandes HG (2004) A novel spray-drying technique to produce low density particles for pulmonary delivery. Int J Pharm 278:187–195. https://doi.org/10.1016/j.ijpharm.2004.03.010
Sun Y, Cui Z, Sun Y, Qin L, Zhang X, Liu Q, Shen X, Yu D, Mao S (2020a) Exploring the potential influence of drug charge on downstream deposition behaviour of DPI powders. Int J Pharm 588:119798. https://doi.org/10.1016/j.ijpharm.2020.119798
Sun Y, Qin L, Liu C, Su J, Zhang X, Yu D, Guo C, Lu H, Li L, Xiong W, Mao S (2020b) Exploring the influence of drug content on DPI powder properties and potential prediction of pulmonary drug deposition. Int J Pharm 575:119000. https://doi.org/10.1016/j.ijpharm.2019.119000
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N (2013) Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm 453:101–125. https://doi.org/10.1016/j.ijpharm.2012.10.043
Torge A, Wagner S, Chaves PS, Oliveira EG, Guterres SS, Pohlmann AR, Titz A, Schneider M, Beck RCR (2017) Ciprofloxacin-loaded lipid-core nanocapsules as mucus penetrating drug delivery system intended for the treatment of bacterial infections in cystic fibrosis. Int J Pharm 527:92–102. https://doi.org/10.1016/j.ijpharm.2017.05.013
Torresan V, Forrer D, Guadagnini A, Badocco D, Pastore P, Casarin M, Selloni A, Coral D, Ceolin M, Fernández van Raap MB, Busato A, Marzola P, Spinelli AE, Amendola V (2020) 4D Multimodal nanomedicines made of nonequilibrium Au–Fe alloy nanoparticles. ACS Nano 14:12840–12853. https://doi.org/10.1021/acsnano.0c03614
Wang A, Huo X, Zhang G, Wang X, Zhang C, Wu C, Rong W, Xu J, Song T (2016) Effect of DSPE-PEG on compound action potential, injury potential and ion concentration following compression in ex vivo spinal cord. Neurosci Lett 620:50–56. https://doi.org/10.1016/j.neulet.2016.03.045
Wang L, Liu Y, Zhao J, Li C, Zhou Y, Du J, Wang Y (2017) In vitro and in vivo evaluation of targeting tumor with folate-based amphiphilic multifunctional stabilizer for resveratrol nanosuspensions. Colloids Surf, B 160:462–472. https://doi.org/10.1016/j.colsurfb.2017.09.056
Wang L, Ma Y, Gu Y, Liu Y, Zhao J, Yan B, Wang Y (2018) Cryoprotectant choice and analyses of freeze-drying drug suspension of nanoparticles with functional stabilisers. J Microencapsul 35:241–248. https://doi.org/10.1080/02652048.2018.1462416
Wistrand-Yuen E, Knopp M, Hjort K, Koskiniemi S, Berg OG, Andersson DI (2018) Evolution of high-level resistance during low-level antibiotic exposure. Nat Commun 9:1599. https://doi.org/10.1038/s41467-018-04059-1
Yan B, Gu Y, Zhao J, Liu Y, Wang L, Wang Y (2019) Self-microemulsion technology for water-insoluble drug delivery. Curr Nanosci 15:576–588. https://doi.org/10.2174/1573413715666190112122107
Yang, X.b., Wang, X.b., Pan, W.s., Xi, R.g., Wang, Y.n., Liu, D., Shi, Y., Jiang, S. (2011) Optimization and characterization of dry powder of fanhuncaoin for inhalation based on selection of excipients. Chem Pharm Bull 59:929–937. https://doi.org/10.1248/cpb.59.929
Yu J, Chan H-K, Gengenbach T, Denman JA (2017) Protection of hydrophobic amino acids against moisture-induced deterioration in the aerosolization performance of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm 119:224–234. https://doi.org/10.1016/j.ejpb.2017.06.023
Zhang Z, Cheng W, Pan Y, Jia L (2020) An anticancer agent-loaded PLGA nanomedicine with glutathione-response and targeted delivery for the treatment of lung cancer. Journal of Materials Chemistry B 8:655–665. https://doi.org/10.1039/C9TB02284H
Zhao J, Wang Y, Ma Y, Liu Y, Yan B, Wang L (2019) Smart nanocarrier based on PEGylated hyaluronic acid for deacetyl mycoepoxydience: high stability with enhanced bioavailability and efficiency. Carbohyd Polym 203:356–368. https://doi.org/10.1016/j.carbpol.2018.09.071
Zhou Y, Du J, Wang L, Wang Y (2017) Nanocrystals technology for improving bioavailability of poorly soluble drugs: a mini-review. Journal of nanoscience and nanotechnology 17:18-28. https://doi.org/10.1166/jnn.2017.13108
Funding
This work was supported by the Shandong Provincial Natural Science Foundation (ZR2019MH105), the projects of “20 Colleges and Universities” in Jinan (2019GXRC068).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, W., Liu, Y., Ding, S. et al. Ciprofloxacin nanocrystals and N-acetylcysteine co-solidified powders for pulmonary drug delivery: development and in vitro and in vivo characterization. J Nanopart Res 24, 41 (2022). https://doi.org/10.1007/s11051-022-05427-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05427-1