Abstract
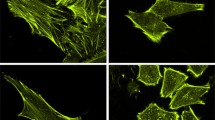
The actin cytoskeleton plays a crucial role in dynamic processes such as cell adhesion and intracellular movement. For cancer cells, both mechanisms are involved in metastasis. Plasmonic photothermal therapy (PPT) is postulated to lessen undesirable damage of both the surrounding healthy cells and tissue. We have examined the actin filaments of HeLa cells treated in vitro with PEGylated gold nanorod (PEGGNR)-based PPT. Our results show that 5 min of PEGGNR-PPT induces irreversible damages on the cytoskeleton network, which initiate with the accumulation of F-actin stress fibers and evolve into cell shrinkage and apoptosis. The PPT mediated by PEGGNRs results in 60.5% of death cells by apoptosis, in which F-actin distribution is significantly accumulated in a reduced area (2.5 times higher than non-treated). Such results suggest that the PEGGNRs could be responsible for compromising the cytoskeleton integrity of HeLa cells while inducing apoptosis.
Graphical abstract






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alabugin A (2019) Near-IR photochemistry for biology: exploiting the optical window of tissue. Photochem Photobiol 95(3):722–732
Aillon K et al (2009) Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev 61(6):457–466
Akiyama Y et al (2012) Conversion of rod-shaped gold nanoparticles to spherical forms and their effect on biodistribution in tumor-bearing mice. Nanoscale Res Lett 7(1):565
Ali MR et al (2017) Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc Natl Acad Sci 114(28):E5655–E5663
Alkilany AM et al (2012) Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev 64(2):190–199
Bhardwaj AK et al (2018) Direct sunlight enabled photo-biochemical synthesis of silver nanoparticles and their bactericidal efficacy: photon energy as key for size and distribution control. J Photochem Photobiol, B 188:42–49
Birukova AA, et al. (2009) Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer. Nanomedicine: Nanotechnology, Biology and Medicine, 2009. 5(1): 30–41
Baronzio GF, Hager ED (2008) Hyperthermia in cancer treatment: a primer. 2008: Springer Science & Business Media
Bhardwaj, A.K., et al. (2021) An overview of silver nano-particles as promising materials for water disinfection. Environ Technol Innovation 23: 101721
Cao J, et al. (2014) Gold nanorod-based localized surface plasmon resonance biosensors: a review. 195: 332-351
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther 1(4):279–293
Cavaliere R et al (1967) Selective heat sensitivity of cancer cells. Biochemical and Clinical Studies Cancer 20(9):1351–1381
Chen H, et al. (2010) Understanding the photothermal conversion efficiency of gold nanocrystals. 2010. 6(20): 2272-2280
Coakley W (1987) Hyperthermia effects on the cytoskeleton and on cell morphology. in Symposia of the Society for Experimental Biology
Desouza M, Gunning PW, Stehn JR (2012) The actin cytoskeleton as a sensor and mediator of apoptosis. BioArchitecture 2(3):75–87
Dominguez R, Holmes KC (2011) Actin structure and function. Annu Rev Biophys 40:169–186
Eghtedari M et al (2009) Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett 9(1):287–291
Eira J et al (2016) The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog Neurobiol 141:61–82
El-Sayed MA, et al. (2013) Tissue distribution and efficacy of gold nanorods coupled with laser induced photoplasmonic therapy in Ehrlich carcinoma solid tumor model. PLoS One 8(10): e76207
Falk M, Issels R (2001) Hyperthermia in oncology. Int J Hyperth 17(1):1–18
Hildebrandt B et al (2002) The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43(1):33–56
Hsu S-W, On K, Tao ARJJotACS (2011) Localized surface plasmon resonances of anisotropic semiconductor nanocrystals. 133(47): 19072–19075
Huang P et al (2011) Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials 32(36):9796–9809
Huang X, El-Sayed MA (2011) Plasmonic photo-thermal therapy (PPTT). Alexandria Journal of Medicine 47(1):1–9
Huang X et al (2008) Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 23(3):217–228
Hurwitz M, Stauffer P (2014) Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. in Seminars in oncology. Elsevier
Issels RD (2008) Hyperthermia adds to chemotherapy. Eur J Cancer 44(17):2546–2554
Jang B, Kim YS, Choi Y (2011) Effects of gold nanorod concentration on the depth-related temperature increase during hyperthermic ablation. Small 7(2):265–270
Josefsen LB, Boyle RW (2012) Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2(9):916
Kaur P et al (2016) Hyperthermia using nanoparticles–promises and pitfalls. Int J Hyperthermia 32(1):76–88
Kelly KL, et al. (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. ACS Publications. 668–677
Kodiha M et al (2015) Off to the organelles-killing cancer cells with targeted gold nanoparticles. Theranostics 5(4):357
Kopwitthaya A, et al. (2010) Biocompatible PEGylated gold nanorods as colored contrast agents for targeted in vivo cancer applications. Nanotechnology, 21(31): 315101
Lankveld DP et al (2011) Blood clearance and tissue distribution of PEGylated and non-PEGylated gold nanorods after intravenous administration in rats. Nanomedicine 6(2):339–349
Lee GY, Lim CT (2007) Biomechanics approaches to studying human diseases. Trends Biotechnol 25(3):111–118
Lepock JR (1982) Involvement of membranes in cellular responses to hyperthermia. Radiat Res 92(3):433–438
Link S, El-Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 103(40):8410–8426
Liu H et al (2011) Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew Chem Int Ed 50(4):891–895
Liu K et al (2015) Biocompatible gold nanorods: one-step surface functionalization, highly colloidal stability, and low cytotoxicity. Langmuir 31(17):4973–4980
Lupo DW, Quack M (1987) IR-laser photochemistry. Chem Rev 87(1):181–216
Mackey MA et al (2014) The most effective gold nanorod size for plasmonic photothermal therapy: theory and in vitro experiments. J Phys Chem B 118(5):1319–1326
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41:189–207
Martensson N, et al. (1980) Core-level binding energies of solid thallium. 19(3): 299-301
McKayed KK, Simpson JCJC (2013) Actin in action: imaging approaches to study cytoskeleton structure and function. 2(4): 715-731
Murphy CJ et al (2005) Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B 109(29):13857–13870
Nehl CL, Hafner JH (2008) Shape-dependent plasmon resonances of gold nanoparticles. J Mater Chem 18(21):2415–2419
Overgaard J (1989) The current and potential role of hyperthermia in radiotherapy. Int J Radiation Oncol * Biology* Physics 16(3): 535–549
Petrova H, et al. (2006) On the temperature stability of gold nanorods: comparison between thermal and ultrafast laser-induced heating. 8(7): 814-821
Plaetzer K et al (2009) Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med Sci 24(2):259–268
Pu Y, et al. (2018) Elucidating the growth mechanism of plasmonic gold nanostars with tunable optical and photothermal properties. 57(14): 8599-8607
Qin Z, et al. (2016) Quantitative comparison of photothermal heat generation between gold nanospheres and nanorods. 6(1): 1-13
Reisler E, Egelman EH (2007) Actin structure and function: what we still do not understand. J Biol Chem 282(50):36133–36137
Schulz F et al (2016) Effective PEGylation of gold nanorods. Nanoscale 8(13):7296–7308
Song CW (1984) Effect of local hyperthermia on blood flow and microenvironment: a review. Can Res 44(10 Supplement):4721s–4730s
Spyratou E et al (2017) Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers 9(12):173
Trendowski M (2014) Exploiting the cytoskeletal filaments of neoplastic cells to potentiate a novel therapeutic approach. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1846(2): 599–616
Ubah CO, Wallace HM (2014) Cancer therapy: targeting mitochondria and other sub-cellular organelles. Current pharmaceutical design, 20(2): 201–222
Vindin H, et al. (2014) Validation of an algorithm to quantify changes in actin cytoskeletal organization 19(3): 354-368
Wang N, Ingber DE (1994) Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J 66(6):2181–2189
Willets KA, Van Duyne RP (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem 58:267–297
Wu Y et al (2018) Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano 12(9):9279–9290
Wust P et al (2002) Hyperthermia in combined treatment of cancer. Lancet Oncol 3(8):487–497
Xu W et al (2012) RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating α(v)β3 expression. Int J Nanomed 7:915–924
Yamaguchi H, Condeelis J (2007) Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1773(5): 642–652
Zou R, et al. (2010) Thermal stability of gold nanorods in an aqueous solution.372(1-3): 177-181
Funding
This work was supported by SEP–CONACyT Mexico, Ciencia Basica Grant No. 1901010 (A1-S-28227).
Author information
Authors and Affiliations
Contributions
The initial work was conducted in Prof. Lal’s laboratory at the UCSD when Prof Santacruz was a visiting faculty at the UCSD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santacruz-Gomez, K., Melendrez, R., Licerio-Ramírez, M. et al. Alterations on HeLa cell actin filaments induced by PEGylated gold nanorod-based plasmonic photothermal therapy. J Nanopart Res 24, 38 (2022). https://doi.org/10.1007/s11051-022-05425-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05425-3