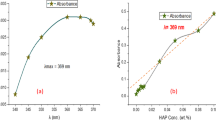
Abstract
Nanosized colloids of iron oxide adsorb heavy metals, enhance the biodegradation of contaminants, and represent a promising technology to clean up contaminated aquifers. Goethite particles for aquifer reclamation were recently synthesized with a coating of humic acids to reduce aggregation. This study investigates the stability and the mobility in porous media of this material as a function of aqueous chemistry, and it identifies the best practices to maximize the efficacy of the related remediation. Humic acid-coated nanogoethite (hydrodynamic diameter ∼90 nm) displays high stability in solutions of NaCl, consistent with effective electrosteric stabilization. However, particle aggregation is fast when calcium is present and, to a lesser extent, also in the presence of magnesium. This result is rationalized with complexation phenomena related to the interaction of divalent cations with humic acid, inducing rapid flocculation and sedimentation of the suspensions. The calcium dose, i.e., the amount of calcium ions with respect to solids in the dispersion, is the parameter governing stability. Therefore, more concentrated slurries may be more stable and mobile in the subsurface than dispersions of low particle concentration. Particle concentration during field injection should be thus chosen based on concentration and proportion of divalent cations in groundwater.

Goethite nanoparticles are used in contaminated site remediation. The particles are stable in monovalent ion solutions due to an adsorbed layer of humic acids. Above a threshold dose of divalent cations, particles aggregate and sediment. High particle/calcium ratios increase colloidal stability. Stability in suspension and transport in porous media correlate well. Delivery into subsurface can be improved by either increasing particle concentration or reducing divalent cation content in the carrier fluid.






Similar content being viewed by others
References
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci Total Environ 407(6):2093–2101. doi:10.1016/j.scitotenv.2008.11.022
Benjamin MM, Sletten RS, Bailey RP, Bennett T (1996) Sorption and filtration of metals using iron-oxide-coated sand. Water Res 30(11):2609–2620. doi:10.1016/S0043-1354(96)00161-3
Bianco C, Tosco T, Sethi R (2016) A 3-dimensional micro-and nanoparticle transport and filtration model (MNM3D) applied to the migration of carbon-based nanomaterials in porous media. J Contam Hydrol 193:10–20. doi:10.1016/j.jconhyd.2016.08.006
Bosch J, Heister K, Hofmann T, Meckenstock RU (2010) Nanosized iron oxide colloids strongly enhance microbial iron reduction. Appl Environ Microb 76(1):184–189. doi:10.1128/Aem.00417-09
Braunschweig J, Bosch J, Meckenstock RU (2013) Iron oxide nanoparticles in geomicrobiology: from biogeochemistry to bioremediation. New Biotechnol 30(6):793–802. doi:10.1016/j.nbt.2013.03.008
Chekli L, Phuntsho S, Tijing LD, Zhou JL, Kim JH, Shon HK (2014) Stability of Fe-oxide nanoparticles coated with natural organic matter under relevant environmental conditions. Water Sci Technol 70(12):2040–2046. doi:10.2166/wst.2014.454
Chen KL, Mylon SE, Elimelech M (2007) Enhanced aggregation of alginate-coated iron oxide (hematite) nanoparticles in the presence of, calcium, strontium and barium cations. Langmuir 23(11):5920–5928. doi:10.1021/la063744k
Domingos RF, Tufenkji N, Wilkinson KJ (2009) Aggregation of titanium dioxide nanoparticles: role of a fulvic acid. Environ Sci Technol 43(5):1282–1286. doi:10.1021/es8023594
Dong HR, Lo IMC (2013a) Influence of calcium ions on the colloidal stability of surface-modified nano zero-valent iron in the absence or presence of humic acid. Water Res 47(7):2489–2496. doi:10.1016/j.watres.2013.02.022
Dong HR, Lo IMC (2013b) Influence of humic acid on the colloidal stability of surface-modified nano zero-valent iron. Water Res 47(1):419–427. doi:10.1016/j.watres.2012.10.013
Elimelech M, Gregory J, Jia X, Williams RA (1995) Particle deposition and aggregation: measurement, modeling, and simulation. Butterworth-Heinemann Ltd, Oxford
Gastone F, Tosco T, Sethi R (2014) Guar gum solutions for improved delivery of iron particles in porous media (part 1): porous medium rheology and guar gum-induced clogging. J Contam Hydrol 166:23–33. doi:10.1016/j.jconhyd.2014.06.013
Hering JG, Morel FMM (1988) Humic-acid complexation of calcium and copper. Environ Sci Technol 22(10):1234–1237. doi:10.1021/Es00175a018
Holthoff H, Schmitt A, FernandezBarbero A, Borkovec M, CabrerizoVilchez MA, Schurtenberger P, HidalgoAlvarez R (1997) Measurement of absolute coagulation rate constants for colloidal particles: comparison of single and multiparticle light scattering techniques. J Colloid Interface Sci 192(2):463–470. doi:10.1006/jcis.1997.5022
Hoss S, Fritzsche A, Meyer C, Bosch J, Meckenstock RU, Totsche KU (2015) Size- and composition-dependent toxicity of synthetic and soil-derived Fe oxide colloids for the nematode Caenorhabditis elegans. Environ Sci Technol 49(1):544–552. doi:10.1021/es503559n
Illes E, Tombacz E (2006) The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J Colloid Interface Sci 295(1):115–123. doi:10.1016/j.jcis.2005.08.003
Keller AA, Wang HT, Zhou DX, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji ZX (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967. doi:10.1021/es902987d
Lee KY, Bosch J, Meckenstock RU (2012) Use of metal-reducing bacteria for bioremediation of soil contaminated with mixed organic and inorganic pollutants. Environ Geochem Hlth 34:135–142. doi:10.1007/s10653-011-9406-2
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46(13):6893–6899. doi:10.1021/Es300839e
Luna M, Gastone F, Tosco T, Sethi R, Velimirovic M, Gemoets J, Muyshondt R, Sapion H, Klaas N, Bastiaens L (2015) Pressure-controlled injection of guar gum stabilized microscale zerovalent iron for groundwater remediation. J Contam Hydrol 181:46–58. doi:10.1016/j.jconhyd.2015.04.007
Maroni P, Ruiz-Cabello FJM, Cardoso C, Tiraferri A (2015) Adsorbed mass of polymers on self-assembled mono layers: effect of surface chemistry and polymer charge. Langmuir 31(22):6045–6054. doi:10.1021/acs.langmuir.5b01103
Meckenstock R, Bosch J (2014) Method for the degradation of pollutants in water and/or soil. U.S. Patent 8,921,091 B2, issued December 30, 2014
Petosa AR, Brennan SJ, Rajput F, Tufenkji N (2012) Transport of two metal oxide nanoparticles in saturated granular porous media: role of water chemistry and particle coating. Water Res 46(4):1273–1285. doi:10.1016/j.watres.2011.12.033
Philippe A, Schaumann GE (2014) Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review. Environ Sci Technol 48(16):8946–8962. doi:10.1021/Es502342r
Raychoudhury T, Tufenkji N, Ghoshal S (2012) Aggregation and deposition kinetics of carboxymethyl cellulose-modified zero-valent iron nanoparticles in porous media. Water Res 46(6):1735–1744. doi:10.1016/j.watres.2011.12.045
Sander S, Mosley LM, Hunter KA (2004) Investigation of interparticle forces in natural waters: effects of adsorbed humic acids on iron oxide and alumina surface properties. Environ Sci Technol 38(18):4791–4796. doi:10.1021/es049602z
Szilagyi I, Trefalt G, Tiraferri A, Maroni P, Borkovec M (2014) Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 10:2479–2502. doi:10.1039/C3SM52132J
Tiraferri A, Borkovec M (2015) Probing effects of polymer adsorption in colloidal particle suspensions by light scattering as relevant for the aquatic environment: an overview. Sci Total Environ 535:131–140. doi:10.1016/j.scitotenv.2014.11.063
Tobler NB, Hofstetter TB, Straub KL, Fontana D, Schwarzenbach RP (2007) Iron-mediated microbial oxidation and abiotic reduction of organic contaminants under anoxic conditions. Environ Sci Technol 41(22):7765–7772. doi:10.1021/Es071128k
Tosco T, Bosch J, Meckenstock RU, Sethi R (2012) Transport of ferrihydrite nanoparticles in saturated porous media: role of ionic strength and flow rate. Environ Sci Technol 46(7):4008–4015. doi:10.1021/es202643c
Tosco T, Gastone F, Sethi R (2014) Guar gum solutions for improved delivery of iron particles in porous media (part 2): iron transport tests and modeling in radial geometry. J Contam Hydrol 166:34–51. doi:10.1016/j.jconhyd.2014.06.014
Tosco T, Papini MP, Viggi CC, Sethi R (2014) Nanoscale zerovalent iron particles for groundwater remediation: a review. J Clean Prod 77:10–21. doi:10.1016/j.jclepro.2013.12.026
Velimirovic M, Simons Q, Bastiaens L (2014) Guar gum coupled microscale ZVI for in situ treatment of CAHs: continuous-flow column study. J Hazard Mater 265:20–29. doi:10.1016/j.jhazmat.2013.11.020
Vindedahl AM, Stemig MS, Arnold WA, Penn RL (2016) Character of humic substances as a predictor for goethite nanoparticle reactivity and aggregation. Environ Sci Technol 50(3):1200–1208. doi:10.1021/acs.est.5b04136
Vindedahl AM, Strehlau JH, Arnold WA, Lee Penn R (2016) Organic matter and iron oxide nanoparticles: aggregation, interactions, and reactivity. Environmental Science: Nano 3:494–505. doi:10.1039/C5EN00215J
Waychunas GA, Kim CS, Banfield JF (2005) Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J Nanopart Res 7(4–5):409–433. doi:10.1007/s11051-005-6931-x
Xu CY, Deng KY, Li JY, Xu RK (2015) Impact of environmental conditions on aggregation kinetics of hematite and goethite nanoparticles. J Nanopart Res 17(10: 394):1–14. doi:10.1007/S11051-015-3198-8
Acknowledgments
This work was partly funded by H2020 EU project “Reground,” G.A. no. 641768. We are grateful to Dr. Rainer Meckenstock (University of Duisburg-Essen, Germany) for providing the goethite particles stock suspension. We thank Fabrizio Bianco and Dr. Adriano Fiorucci (Politecnico di Torino) for their chemical analyses on tap water.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Electronic Supplementary Material
ESM 1
Electrophoretic mobility of humic acid-coated goethite nanoparticle suspensions as a function of ionic strength. Examples of raw data from aggregation experiments. Apparent aggregation rate as a function of slurry concentration in the presence of 0.7 mM CaCl2. Additional results of sedimentation experiments at varying concentration of CaCl2 and MgCl2. Pictures of sedimentation vials for different doses of calcium. Representative results of sedimentation experiments of suspensions from which most of the unadsorbed humic acid were removed by filtration. Breakthrough curves of humic acid-coated goethite nanoparticles (total solid content of 1.70 g/L) in silica sand in 10 mM NaCl. Pictures of the column during transport tests carried out in 1.5 mM CaCl2 and 10 g/L solid content. Pictures of sedimentation vials for different dilutions in tap water. Results of transport tests conducted with suspensions diluted with tap water (PDF). (PDF 1658 kb)
Rights and permissions
About this article
Cite this article
Tiraferri, A., Saldarriaga Hernandez, L.A., Bianco, C. et al. Colloidal behavior of goethite nanoparticles modified with humic acid and implications for aquifer reclamation. J Nanopart Res 19, 107 (2017). https://doi.org/10.1007/s11051-017-3814-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3814-x