Abstract
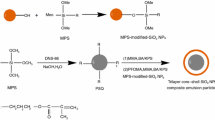
Siloxane @ poly (methylacrylic acid) core-shell microparticles with functional groups were prepared by a facile hydrolysis-condensation method in this work. Three different silane coupling agents 3-methacryloxypropyltrimethoxysilane (MPS), 3-triethoxysilylpropylamine (APTES), and 3-glycidoxypropyltrimethoxysilane (GPTMS) were added along with tetraethoxysilane (TEOS) into the polymethylacrylic acid (PMAA) microparticle ethanol dispersion to form the Si@PMAA core-shell microparticles with different functional groups. The core-shell structure and the surface special functional groups of the resulting microparticles were measured by transmission electron microscopy and FTIR. The sizes of these core-shell microparticles were about 350–400 nm. The corresponding preparation conditions and mechanism were discussed in detail. This hydrolysis-condensation method also could be used to functionalize other microparticles which contain active groups on the surface. Meanwhile, the Si@PMAA core-shell microparticles with carbon-carbon double bonds and amino groups have further been applied to prepare hydrophobic coatings.







Similar content being viewed by others
References
Bai F, Yang XL, Li R, Huang B, Huang WQ (2006) Monodisperse hydrophilic polymer microspheres having carboxylic acid groups prepared by distillation precipitation polymerization. Polymer 47:5775–5784
Che JF, Xiao YH, Wang X, Pan AB, Yuan W, Wu XD (2007) Grafting polymerization of polyacetal onto nano-silica surface via bridging isocyanate. Surf Coat Technol 201:4578–4584
Chen JP, Zhu XS (2016) Magnetic solid phase extraction using ionic liquid-coated core-shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of Rhodamine B in food samples. Food Chem 200:10–15
Fedeyko JM, Rimer JD, Lobo RF, Vlachos DG (2004) Spontaneous formation of silica nanoparticles in basic solutions of small tetraalkylammonium cations. J Phys Chem B 108:12271–12275
Fleming MS, Mandal TK, Walt DR (2001) Nanosphere-microsphere assembly: methods for core-shell materials preparation. Chem Mat 13:2210–2216
Gao N, Yang T, Liu T, Zou Y, Jiang J (2015) Graphene oxide wrapped individual silver nanocomposites with improved stability for surface-enhanced Raman scattering. RSC Adv 5:55801–55807
Heng CN, Liu MY, Wang K, Deng FJ, Huang HY, Wan Q, Hui JF, Zhang XY, Wei Y (2015) Biomimic preparation of highly dispersible silica nanoparticles based polymer nanocomposites. Ceram Int 41:15075–15082
Kang H, Kim M, Park KH (2015) Effective immobilization of gold nanoparticles on core-shell thiol-functionalized GO coated TiO2 and their catalytic application in the reduction of 4-nitrophenol. Appl Catal A-Gen 502:239–245
Knight MW, Halas NJ (2008) Nanoshells to nanoeggs to nanocups: optical properties of reduced symmetry core-shell nanoparticles beyond the quasistatic limit. New J Phys 10:10
Kopanja L, Kralj S, Zunic D, Loncar B, Tadic M (2016) Core-shell superparamagnetic iron oxide nanoparticle (SPION) clusters: TEM micrograph analysis, particle design and shape analysis. Ceram Int 42:10976–10984
Lee SG, Ham DS, Lee DY, Bong H, Che K (2013) Transparent superhydrophobic/translucent superamphiphobic coatings based on silica-fluoropolymer hybrid nanoparticles. Langmuir 29:15051–15057
Lin JB, Chen HL, Ji Y, Zhang Y (2012) Functionally modified monodisperse core-shell silica nanoparticles: silane coupling agent as capping and size tuning agent. Colloid Surf A-Physicochem Eng Asp 411:111–121
Liu JX, Yang KG, Qu YY, Li SW, Wu Q, Liang Z, Zhang LH, Zhang YK (2015) An efficient approach to prepare boronate core-shell polymer nanoparticles for glycoprotein recognition via combined distillation precipitation polymerization and RAFT media precipitation polymerization. Chem Commun 51:3896–3898
Marini M, Pourabbas B, Pilati F, Fabbri P (2008) Functionally modified core-shell silica nanoparticles by one-pot synthesis. Colloid Surf A-Physicochem Eng Asp 317:473–481
Moreno YP, Cardoso MB, Ferrao MF, Moncada BA, Dos Santos JHZ (2016) Effect of SiCl4 on the preparation of functionalized mixed-structure silica from monodisperse sol-gel silica nanoparticles. Chem Eng J 292:233–245
Murugan E, Jebaranjitham JN (2015) Dendrimer grafted core-shell Fe3O4-polymer magnetic nanocomposites stabilized with AuNPs for enhanced catalytic degradation of Rhodamine B—a kinetic study. Chem Eng J 259:266–276
Rigo VA, De Lara LS, Miranda CR (2014) Energetics of formation and hydration of functionalized silica nanoparticles: an atomistic computational study. Appl Surf Sci 292:742–749
Shahnaz K, Nazanin F, Mirabdullah SS (2015) Design and fabrication of core-shell gold coated Fe3O4 magnetic nanoparticles as cancer cells targeting agent. Res J Biotechnol 10:27–33
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275:496–502
Strasser P et al (2010) Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat Chem 2:454–460
Tang QQ, Chen MM, Yang CY, Wang WQ, Bao H, Wang GC (2015) Enhancing the energy density of asymmetric stretchable supercapacitor based on wrinkled CNT@MnO2 cathode and CNT@polypyrrole anode. ACS Appl Mater Interfaces 7:15303–15313
Tang F, Wang C, Li LD (2016) Controlled fabrication of fluorescent Au@PAA nanocomposites. Colloid Surf A-Physicochem Eng Asp 494:95–100
Yola ML, Eren T, Atar N, Wang SB (2014) Adsorptive and photocatalytic removal of reactive dyes by silver nanoparticle-colemanite ore waste. Chem Eng J 242:333–340
Zhang Y, Chen HL, Wen YJ, Yuan YB, Wu W, Liu C (2014) Tunable wettability of monodisperse core-shell nano-SiO2 modified with poly (methylhydrosiloxane) and allyl-poly (ethylene glycol). Colloid Surf A-Physicochem Eng Asp 441:16–24
Zhao ZB, Zhang DM, Meng YF, Tai L, Jiang Y (2016) One-pot fabrication of fluoride-silica@silica raspberry-like nanoparticles for superhydrophobic coating. Ceram Int 42:14601–14608
Zou YJ, Wang QY, Jiang DD, Xiang CL, Chu HL, Qiu SJ, Zhang HZ, Xu F, Sun LX, Liu SS (2016) Pd-doped TiO2@polypyrrole core-shell composites as hydrogen-sensing materials. Ceram Int 42:8257–8262
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) with grant number 21174029, the Industry Academia Cooperation Innovation Fund of Jiangsu Province with grant number BY2014127-07, and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) with grant number 1107047002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information Figure S1 shows the wide-area TEM images of core-shell microparticles with different cores. Figure S2 shows the wide-area SEM images of Si@PMAA core-shell microparticles with carbon-carbon double bonds and amino groups. Figure S3 shows the TEM images of Si@PMAA core-shell microparticles with carbon-carbon double bonds and amino groups, the volumetric ratio of TEOS and silane coupling agents was 1:2. Figure S4 shows the TEM images of Si@PMAA core-shell microparticles prepared via the hydrolysis-condensation of TEOS and GPTMS under different conditions. Figure S5 shows the FTIR spectra of @PMAA core-shell microparticles prepared via the hydrolysis-condensation of TEOS and GPTMS under acidic and basic conditions. Figure S6 shows the practical application of Si@PMAA core-shell microparticles with amino groups.
ESM 1
(DOCX 52895 kb)
Rights and permissions
About this article
Cite this article
Zhao, ZB., Tai, L., Zhang, DM. et al. Facile fabrication of siloxane @ poly (methylacrylic acid) core-shell microparticles with different functional groups. J Nanopart Res 19, 73 (2017). https://doi.org/10.1007/s11051-017-3777-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3777-y