Abstract
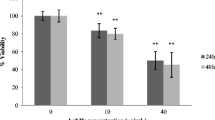
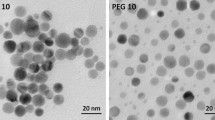
Silver nanoparticles (AgNPs) are among the most widely used nanomaterials in medical and consumer products. However, safety in the uses of AgNPs is still controversial. The toxicity of AgNPs toward various cell types has been reported to depend on the surface properties of the nanoparticles. In this study, the effect of AgNPs with the average size of 5–15 nm on the viability of the CCD-986SK human normal skin fibroblast cell line and A375 human malignant melanoma cell line was evaluated. Comparative toxicity studies, based on MTT assay, were performed by using either sodium alginate or poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA) as capping agent in the nanoparticle preparation. The cytotoxicity tests revealed that AgNO3 alone was highly toxic to both cell types while both alginate and PSSMA alone were not toxic. AgNPs capped with alginate were selectively toxic to the cancer cell line but not to the normal cell line while AgNPs capped with PSSMA were toxic to both cancer and normal cell lines. Judging from the 50 % inhibition concentration (IC50), it was found that the cancer cell line was more sensitive to AgNPs than the normal cell line. Study on the mode of cell death by annexin V and propidium iodide staining revealed that AgNPs induced more apoptotic cell death (84–90 %) than necrosis (8–12 %) in the skin cancer cell line. These results suggest that the toxicity of AgNPs depended on the type of capping agent and the type of cell line.







Similar content being viewed by others
References
Albers CE, Hofstetter W, Siebenrock KA, Landmann R, Klenke FM (2013) In vitro cytotoxicity of silver nanoparticles on osteolasts and osteoclasts at antibacterial concentrations. Nanotoxicology 7:30–36
Arjmandi N, Van Roy W, Lagae L, Borgh G (2012) Measuring the electric charge and zeta potential of nanometer-sized objects using pyramidal-shaped nanopores. Anal Chem 84:8490–8496
Arora S, Tyagi N, Bhardwaj A, Rusu L, Palanki R, Vig K, Singh SR, Singh AP, Palanki S, Miller ME, Carter JE, Singh S (2015) Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: potential for prevention of skin carcinogenesis. Nanomed-Nanotechnol Biol Med 11:1265–1275
AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290
Awasthi KK, Awasthi A, Kumar N, Roy P, Awasthi K, John PJ (2013) Silver nanoparticle induced cytotoxicity, oxidative stress, and DNA damage in CHO cells. J Nanopart Res 15:1898. doi:10.1007/s11051-013-1898-5
Belizario J, Alves J, Occhiucci JM, Malpartida MG, Sesso A (2007) A mechanistic view of mitochondrial death decision pores. Braz J Med Biol Res 40:1011–1024
Chen T, Wong YS, Zheng W, Bai Y, Huang L (2008) Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloid Surface B 67:26–31
Christensen FM, Johnston HJ, Stone V, Aitken RJ, Hankin S, Peters S, Aschberger K (2010) Nano-silver—feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology 4:284–295
Cohen MS, Stern JM, Vanni AJ, Kelly RS, Baumgart E, Field D, Libertino JA, Summerhayes IC (2007) In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg Infect 8:397–403
Dibrov P, Dzioba J, Gosink K, Hase C (2002) Chemiosmotic mechanism of antibacterial activity of Ag+ in Vibrio cholera. Antimicrob Agents Chemother 46:2668–2670
El-Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Techol 45:283–287
Feng Q, Wu J, Chen G, Cui F, Kim T, Kim J (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Foldbjerg R, Dang DA, Autrup H (2011) Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol 85:743–750
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43:9216–9222
Han JW, Gurunathan S, Jeong JK, Choi YJ, Kwon DN, Park JK, Kim JH (2014) Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res Lett 9:459
Ip M, Lui SL, Poon VK, Lung I, Burd A (2006) Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol 55:59–63
Jones CM, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Jones SA, Bowler PG, Walker M, Parsons D (2004) Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair Regen 12:288–294
Jung W, Koo H, Kim K, Shin S, Kim S, Park Y (2008) Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol 74:2171–2178
Kim S, Ryu DY (2013) Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosos in cultured cells and animal tissues. J Appl Toxicol 33:78–89
Lee HY, Park HK, Lee YM, Kim K, Park SB (2007) A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem Commun (Camb) 28:2959–2961
Lima RD, Seabra AB, Durán N (2012) Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol 32:867–879
Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB (2010) Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4:319–330
Moutin MJ, Abramson JJ, Salama G, Dupont Y (1989) Rapid Ag+-induced release of Ca2+ from sarcoplasmic reticulum vesicles of skeletal muscle: a rapid filtration study. Biochim Biophys Acta 984:289–292
Mukherjee SG, O’Claonadh N, Casey A, Chambers G (2012) Comparative in vitro cytotoxicity study of silver nanoparticles on two mammalian cell lines. Toxicol in Vitro 26:238–251
Orrenius S, McCabe MJ Jr, Nicotera P (1992) Ca2+-dependent mechanisms of cytotoxicity and programmed cell death. Toxicol Lett 64:357–364
Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishnan A, Sokolov J, Ulman A, Rafailovich M (2006) Adverse effects of citrate/gold nanoparticles on human dermal fibroblast. Small 2:766–773
Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C (2007) Synthesis and study of silver nanoparticles. J Chem Educ 84:322–325
Song KC, Lee SM, Park TS, Lee BS (2009) Preparation of colloidal silver nanoparticles by chemical reduction method. Korean J Chem Eng 26:153–155
Stickler DJ (2000) Biomaterials to prevent nosocomial infections: is silver the gold standard? Curr Opin Infect Dis 13:389–393
Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH (2007) Functional finishing of carbon fabrics using silver nanoparticles. Nanosci Nanotechnol 7:1893–1897
Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ (2002) Design of biodegradable particles for protein delivery. J Control Release 78:15–24
Wallace WE, Keane MJ, Murray DK, Chisholm WP, Maynard AD, Ong TM (2006) Phospholipid lung surfactant and nanoparticle surface toxicity: lessons from diesel soots and silicate dusts. J Nanopart Res 9:23–38
Wiesner MR, Lowry GV, Alvarez PJJ (2006) Assessing the risks of manufactured nanomaterials. Environ Sci Technol 40:4337–4345
Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van De Meent D, Dekkers S, De Jong WH, Van Zijverden M, Sips AJAM, Geertsma RE (2009) Nano-silver—a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3:109–138
Yang H, Liu C, Yang D, Zhang H, Xi Z (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29:69–78
Acknowledgments
This research was financially supported by Chulalongkorn University, Ratchadaphiseksomphot Endowment Fund (grant no. GF_58_05_61_01), and Royal Thai Government budget (grant no. GBR_BSS_100_59_61_07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig. 1
UV-Vis absorbance spectra of AgNPs solutions capped with Alginate (0.04–4.56 mM) using NaBH4 at (A) 3.8 mM, (B) 19 mM and (C) 38 mM. Each line represents spectra of the AgNPs solution prepared at different molar ratio of AgNO3 to alginate to NaBH4 (GIF 127 kb)
Supplementary Fig. 2
UV-Vis absorbance spectra of AgNPs solutions capped with PSSMA (0.04–4.56 mM) using NaBH4 at (A) 3.8 mM, (B) 19 mM and (C) 38 mM. Each line represents spectra of the AgNPs solution prepared at different molar ratio of AgNO3 to PSSMA to NaBH4 (GIF 124 kb)
Supplementary Fig. 3
Size distribution plots of AgNPs prepared at different ratios of the precursors (GIF 160 kb)
Supplementary Fig. 4
Cell viability of human skin normal cell (CCD-986SK) (♦) and human skin cancer cell (A375) (▲) after treatment with (a) AgNO3, (b) alginate and (c) PSSMA for 72 h (GIF 168 kb)
Supplementary Fig. 5
Cytotoxicity of AgNPs capped with (a) alginate and (b) PSSMA at (1) 0.23 mM, (2) 1.14 mM and (3) 4.56 mM to human skin cancer cell (A375) after exposure for 24, 48, and 72 h (GIF 188 kb)
Supplementary Fig. 6
Annexin V-PI staining of CCD-986SK cells treated with 600 μg/mL AgNPs for 72 h. A: untreated and unstained cells, B: untreated cells stained with Annexin V and PI, C: cells treated with AgNPs capped with alginate and stained with Annexin V and PI, D: cells treated with AgNPs capped with Copss and stained with Annexin V and PI, E: cells treated with 1 μg/mL doxorubicin and stained with Annexin V and PI (positive control). Quadrant 1: necrosis, quadrant 2: late apoptosis, quadrant 3: live cell, quadrant 4: early apoptosis (GIF 227 kb)
Supplementary Fig. 7
Annexin V-PI staining of A375 cells treated with 600 μg/mL AgNPs for 72 h. A: untreated and unstained cells, B: untreated cells stained with Annexin V and PI, C: cells treated with AbNPs capped with alginate and stained with Annexin V and PI, D: cells treated with AgNPs capped with Copss and stained with Annexin V and PI, E: cells treated with 1 μg/mL doxorubicin and stained with Annexin V and PI (positive control). Quadrant 1: necrosis, quadrant 2: late apoptosis, quadrant 3: live cell, quadrant 4: early apoptosis (GIF 215 kb)
Rights and permissions
About this article
Cite this article
Netchareonsirisuk, P., Puthong, S., Dubas, S. et al. Effect of capping agents on the cytotoxicity of silver nanoparticles in human normal and cancer skin cell lines. J Nanopart Res 18, 322 (2016). https://doi.org/10.1007/s11051-016-3624-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3624-6