Abstract
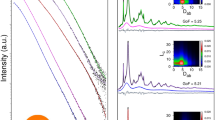
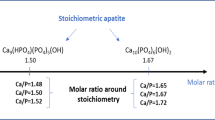
Rapid calcium (Ca) dissolution from nanostructured Ca phosphate and carbonate (CaCO3) powders may allow them to be absorbed in much higher fraction in humans. Nanosized Ca phosphate and CaCO3 made by flame-assisted spray pyrolysis were characterized by nitrogen adsorption, X-ray diffraction (XRD), Raman spectroscopy, and transmission electron microscopy. As-prepared nanopowders contained both CaCO3 and CaO, but storing them under ambient conditions over 130 days resulted in a complete transformation into CaCO3, with an increase in both crystal and particle sizes. The small particle size could be stabilized against such aging by cation (Mg, Zn, Sr) and anion (P) doping, with P and Mg being most effective. Calcium phosphate nanopowders made at Ca:P ≤ 1.5 were XRD amorphous and contained γ-Ca2P2O7 with increasing hydroxyapatite content at higher Ca:P. Aging of powders with Ca:P = 1.0 and 1.5 for over 500 days gradually increased particle size (but less than for CaCO3) without a change in phase composition or crystallinity. In 0.01 M H3PO4 calcium phosphate nanopowders dissolved ≈4 times more Ca than micronsized compounds and about twice more Ca than CaCO3 nanopowders, confirming that nanosizing and/or amorphous structuring sharply increases Ca powder dissolution. Because higher Ca solubility in vitro generally leads to greater absorption in vivo, these novel FASP-made Ca nanostructured compounds may prove useful for nutrition applications, including supplementation and/or food fortification.









Similar content being viewed by others
References
Ali M, Winterer M (2010) ZnO nanocrystals: surprisingly ‘alive’. Chem Mater 22:85–91
Brennan MJ, Duncan WE, Wartofsky L, Butler VM, Wray HL (1991) In vitro dissolution of calcium carbonate preparations. Calcif Tissue Int 49:308–312
Camenzind A, Strobel R, Pratsinis SE (2005) Cubic or monoclinic Y2O3: Eu3+ nanoparticles by one step flame spray pyrolysis. Chem Phys Lett 415:193–197
Cheary RW, Coelho A (1992) A fundamental parameters approach to X-ray line profile fitting. J Appl Crystallogr 25:109–121
Chen SF, Yu SH, Jiang J, Li FQ, Liu YK (2006) Polymorph discrimination of CaCO3 mineral in an ethanol/water solution: formation of complex vaterite superstructures and aragonite rods. Chem Mater 18:115–122
Chen HS, Chang JH, Wu JSB (2008) Calcium bioavailability of nanonized pearl powder for adults. J Food Sci 73:H246–H251
Chou L, Garrels RM, Wollast R (1989) Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem Geol 78:269–282
European Commission (1998) Report on osteoporosis in the European Community: action for prevention. Office for official publications for the European Commission, Luxembourg
Davis KJ, Dove PM, de Yoreo JJ (2000) The role of Mg2+ as an impurity in calcite growth. Science 290:1134–1137
de Leeuw NH (2002) Molecular dynamics simulations of the growth inhibiting effect of Fe2+, Mg2+, Cd2+, and Sr2+ on calcite crystal growth. J Phys Chem B 106:5241–5249
Dheilly RM, Tudo J, Queneudec M (1998) Influence of climatic conditions on the carbonation of quicklime. J Mater Eng Perform 7:789–795
Dickmann RS, Strasburg GM, Romsos DR, Wilson LA, Lai GH, Huang H (2016) Particle size, surface area, and amorphous content as predictors of solubility and bioavailability for five commercial sources of ferric orthophosphate in ready-to-eat cereal. Nutrients 8:14 pp
Doman RC, Barr JB, McNally RN, Alper AM (1963) Phase equilibria in the system CaO-MgO. J Am Ceram Soc 46:313–316
Dorozhkin SV (2007) Calcium orthophosphates. J Mater Sci 42:1061–1095
Economou ED, Evmiridis NP, Vlessidis AG (1996) Dissolution kinetics of CaCO3 in powder form and influence of particle size and pretreatment on the course of dissolution. Ind Eng Chem Res 35:465–474
Frear GL, Deese EF, Lefforge JW (1944) Calcium metaphosphate—effect of impurities on fusibility, citrate solubility, and hygroscopicity. Ind Eng Chem 36:835–840
Goldsmith JR, Heard HC (1961) Subsolidus phase relations in the system CaCO3-MgCO3. J Geol 69:45–74
Gomez-Villalba LS, Lopez-Arce P, Alvarez de Buergo M, Fort R (2011) Structural stability of a colloidal solution of Ca(OH)2 nanocrystals exposed to high relative humidity conditions. Appl Phys A-Mater Sci Process 104:1249–1254
Gueguen L, Pointillart A (2000) The bioavailability of dietary calcium. J Am Coll Nutr 19:119S–136S
Hansen C, Werner E, Erbes HJ, Larrat V, Kaltwasser JP (1996) Intestinal calcium absorption from different calcium preparations: influence of anion and solubility. Osteoporos Int 6:386–393
Hausner DB, Reeder RJ, Strongin DR (2007) Humidity-induced restructuring of the calcite surface and the effect of divalent heavy metals. J Colloid Interface Sci 305:101–110
Haynes WM (2015–2016) CRC Handbook of Chemistry and Physics
Heaney RP, Recker RR, Weaver CM (1990) Absorbability of calcium sources—the limited role of solubility. Calcif Tissue Int 46:300–304
Heaney RP, Dowell MS, Barger-Lux MJ (1999) Absorption of calcium as the carbonate and citrate salts, with some observations on method. Osteoporos Int 9:19–23
Hilty FM, Teleki A, Krumeich F, Buchel R, Hurrell RF, Pratsinis SE, Zimmermann MB (2009) Development and optimization of iron- and zinc-containing nanostructured powders for nutritional applications. Nanotechnol 20:475101
Hilty FM, Arnold M, Hilbe M, Teleki A, Knijnenburg JTN, Ehrensperger F, Hurrell RF, Pratsinis SE, Langhans W, Zimmermann MB (2010) Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat Nanotechnol 5:374–380
Hilty FM, Knijnenburg JTN, Teleki A, Krumeich F, Hurrell RF, Pratsinis SE, Zimmermann MB (2011) Incorporation of Mg and Ca into nanostructured Fe2O3 improves Fe solubility in dilute acid and sensory characteristics in foods. J Food Sci 76:N2–N10
Huang S, Chen JC, Hsu CW, Chang WH (2009) Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnol 20:375102
Huber M, Stark WJ, Loher S, Maciejewski M, Krumeich F, Baiker A (2005) Flame synthesis of calcium carbonate nanoparticles. Chem Commun. doi:10.1039/B411725E
Imel EA, DiMeglio LA, Burr DB (2014) Chapter 16—Metabolic bone diseases. In: Burr DB, Allen MR (eds) Basic and applied bone biology. Academic Press, San Diego, pp 317–344
Institute of Medicine (1997) Dietary reference intake for calcium, phosphorus, magnesium, vitamin D and fluoride. National Academy Press, Washington
Jossen R, Pratsinis SE, Stark WJ, Madler L (2005) Criteria for flame-spray synthesis of hollow, shell-like, or inhomogeneous oxides. J Am Ceram Soc 88:1388–1393
Knijnenburg JTN, Hilty FM, Krumeich F, Zimmermann MB, Pratsinis SE (2013) Multimineral nutritional supplements in a nano-CaO matrix. J Mater Res 28:1129–1138
Knijnenburg JTN, Seristatidou E, Hilty FM, Krumeich F, Deligiannakis Y (2014) Proton-promoted iron dissolution from nanoparticles and the influence by the local iron environment. J Phys Chem C 118:24072–24080
Loher S, Stark WJ, Maciejewski M, Baiker A, Pratsinis SE, Reichardt D, Maspero F, Krumeich F, Gunther D (2005) Fluoro-apatite and calcium phosphate nanoparticles by flame synthesis. Chem Mater 17:36–42
Loste E, Wilson RM, Seshadri R, Meldrum FC (2003) The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J Cryst Growth 254:206–218
Lu H, Smirniotis PG, Ernst FO, Pratsinis SE (2009) Nanostructured Ca-based sorbents with high CO2 uptake efficiency. Chem Eng Sci 64:1936–1943
Madler L, Kammler HK, Mueller R, Pratsinis SE (2002) Controlled synthesis of nanostructured particles by flame spray pyrolysis. J Aerosol Sci 33:369–389
Mao ZQ, Maeno Y, Fukazawa H (2000) Crystal growth of Sr2RuO4. Mater Res Bull 35:1813–1824
Margolis HC, Moreno EC (1992) Kinetics of hydroxyapatite dissolution in acetic, lactic, and phosphoric acid solutions. Calcif Tissue Int 50:137–143
Marie PJ, Ammann P, Boivin G, Rey C (2001) Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int 69:121–129
Meiron OE, Bar-David E, Aflalo ED, Shechter A, Stepensky D, Berman A, Sagi A (2011) Solubility and bioavailability of stabilized amorphous calcium carbonate. J Bone Miner Res 26:364–372
Meyer K (1998) Characterisation of the structure of binary calcium ultraphosphate glasses by infrared and Raman spectroscopy. Phys Chem Glasses 39:108–117
Morse JW, Arvidson RS, Luttge A (2007) Calcium carbonate formation and dissolution. Chem Rev 107:342–381
Mueller R, Madler L, Pratsinis SE (2003) Nanoparticle synthesis at high production rates by flame spray pyrolysis. Chem Eng Sci 58:1969–1976
Nordin BEC (1997) Calcium and osteoporosis. Nutrition 13:664–686
Orchard TS, Larson JC, Alghothani N, Bout-Tabaku S, Cauley JA, Chen Z, LaCroix AZ, Wactawski-Wende J, Jackson RD (2014) Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr 99:926–933
Pak CYC, Avioli LV (1988) Factors affecting absorbability of calcium from calcium salts and food. Calcif Tissue Int 43:55–60
Pak CYC, Poindexter J, Finlayson B (1989) A model system for assessing physicochemical factors affecting calcium absorbability from the intestinal tract. J Bone Miner Res 4:119–127
Pemberton JE, Latifzadeh L, Fletcher JP, Risbud SH (1991) Raman spectroscopy of calcium phosphate glasses with varying CaO modifier concentrations. Chem Mater 3:195–200
Puech J, Heughebaert JC, Montel G (1982) A new mode of growing apatite crystallites. J Cryst Growth 56:20–24
Recker RR (1985) Calcium absorption and achlorhydria. N Engl J Med 313:70–73
Rohner F, Ernst FO, Arnold M, Hilbe M, Biebinger R, Ehrensperger F, Pratsinis SE, Langhans W, Hurrell RF, Zimmermann MB (2007) Synthesis, characterization, and bioavailability in rats of ferric phosphate nanoparticles. J Nutr 137:614–619
Rudin T, Pratsinis SE (2012) Homogeneous iron phosphate nanoparticles by combustion of sprays. Ind Eng Chem Res 51:7891–7900
Rudin T, Wegner K, Pratsinis SE (2011) Uniform nanoparticles by flame-assisted spray pyrolysis (FASP) of low cost precursors. J Nanopart Res 13:2715–2725
Shih SM, Ho CS, Song YS, Lin JP (1999) Kinetics of the reaction of Ca(OH)2 with CO2 at low temperature. Ind Eng Chem Res 38:1316–1322
Silaban A, Harrison DP (1995) High temperature capture of carbon dioxide: Characteristics of the reversible reaction between CaO(s) and CO2(g). Chem Eng Commun 137:177–190
Sipponen P, Harkonen M (2010) Hypochlorhydric stomach: a risk condition for calcium malabsorption and osteoporosis? Scand J Gastroenterol 45:133–138
Strobel R, Pratsinis SE (2007) Flame aerosol synthesis of smart nanostructured materials. J Mater Chem 17:4743–4756
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York
Sun LM, Chow LC, Frukhtbeyn SA, Bonevich JE (2010) Preparation and properties of nanoparticles of calcium phosphates with various Ca/P ratios. J Res Natl Inst Stand Technol 115:243–255
Tsugawa N, Yamabe T, Takeuchi A, Kamao M, Nakagawa K, Nishijima K, Okano T (1999) Intestinal absorption of calcium from calcium ascorbate in rats. J Bone Miner Metab 17:30–36
Vaisman N, Shaltiel G, Daniely M, Meiron OE, Shechter A, Abrams SA, Niv E, Shapira Y, Sagi A (2014) Increased calcium absorption from synthetic stable amorphous calcium carbonate: double-blind randomized crossover clinical trial in postmenopausal women. J Bone Miner Res 29:2203–2209
Weaver CM, Gallant KMH (2014) Chapter 14—Nutrition. In: Burr DB, Allen MR (eds) Basic and applied bone biology. Academic Press, San Diego, pp 283–297
Willis RB, Allen PR (1999) Measurement of amorphous ferric phosphate to assess iron bioavailability in diets and diet ingredients. Analyst 124:425–430
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143
Yang LC, Kim P, Meyer HM, Agnihotri S (2009) Aging of nanocarbons in ambient conditions: Probable metastability of carbon nanotubes. J Colloid Interface Sci 338:128–134
Acknowledgments
The authors would like to thank Seline Staub for her assistance with production and analysis of the carbonate-containing powders. Also, the authors would like to thank Burgerstein Vitamins, Switzerland, for donating Ca compounds used in these studies. TEM measurements were taken at ScopeM (ETH Zurich). This work was financially supported by ETH Research Grant ETH-06 10-1 and the Swiss South African Joint Research Programme (project number IZLSZ3_149090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lidija Posavec and Jesper T.N. Knijnenburg contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Posavec, L., Knijnenburg, J.T.N., Hilty, F.M. et al. Dissolution and storage stability of nanostructured calcium carbonates and phosphates for nutrition. J Nanopart Res 18, 310 (2016). https://doi.org/10.1007/s11051-016-3608-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3608-6