Abstract
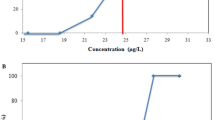
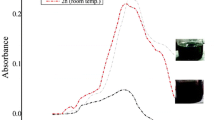
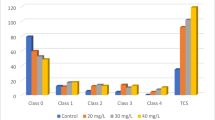
Gold nanoparticles (AuNPs) have attracted a lot of attention due to their usage in consumer- and therapy-based biomedical applications. These particles are frequently the medium-sized particles within the range of 10–50 nm. A number of scientific reports have addressed the cytotoxic potential of these NPs. However, their genotoxic potential with respect to reproductive aspects remains unclear. For assessment of safety and risks associated with AuNPs to female reproductive system, adult female zebrafish (Danio rerio) were exposed in vivo to 20 μg/g/day of AuNPs of two different sizes. AuNPs of 15 nm (type I) and 47 nm (type II) in diameters were administered orally to female zebrafish for a period of 28 days (chronic). The ability of these AuNPs to gain access to female reproductive organs was confirmed by their accumulation pattern through inductive coupled plasma mass spectroscopy. Gonads were assessed for changes in ovarian morphology at histopathological level followed by the confirmation of bioaccumulation of AuNPs using transmission electron microscopy. Using comet assay, strand breaks in DNA of ovarian cells were investigated. Chronic exposure to type I and II AuNPs showed distinctive patterns of bioaccumulation in ovaries. Interestingly, accumulated NPs resulted in gross cellular alterations in different cell types of ovarian tissue. Comet assay analysis revealed extensive number of strand breaks in ovarian cells from the NP exposed fishes. In conclusion, AuNPs ranging between 10 and 50 nm are capable of gaining access to ovaries of zebrafish and potential enough to cause strand breaks in ovarian cells. The findings of the present study highlight the adverse effects of these NPs to female reproductive system. It opens up further avenues for research on effects of these NPs on F1 generation descending from the exposed fishes.






Similar content being viewed by others
References
Abdelhalim MAK, El-Toni AM (2012) Optimization of a citrate method for synthesizing GNPs of controllable particle size and monodispersity: physical studies. Open Access Scientific Reports 1(5):1–5
Ajnai G, Chiu A, Kan T, Cheng CC, Tsai TH, Chang J (2014) Trends of gold nanoparticle-based drug delivery system in cancer therapy. J Exp Clin Med 6(6):172–178
Bourrachot S, Brion F, Pereira S, Floriani M, Camilleri V, Cavalié I, Adam-Guillermin C (2014) Effects of depleted uranium on the reproductive success and F1 generation survival of zebrafish (Danio rerio). Aquat Toxicol 154:1–11
Braunbeck T, Storch V (1992) Senescence of hepatocytes isolated from rainbow trout (Oncorhynchus mykiss) in primary culture. Protoplasma 170(3–4):138–159
Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Frangioni JV (2007) Renal clearance of quantum dots. Nat Biotechnol 25(10):1165–1170
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1(3):325–327
Dayal N, Thakur M, Soparkar A, Doctor M, Patil P, Joshi DS (2016a) Effective method to deliver test substance in adult zebrafish. Int J Adv Res 4(7):543–551
Dayal N, Thakur M, Patil P, Swain N, Joshi DS (2016b) Effects of subacute exposure to gold nanoparticles on germ cells of zebrafish (Danio rerio): an in vivo study. MGM J Med Sci 3(1):1–6
De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE (2008) Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29(12):1912–1919
Doak SH, Griffiths SM, Manshian B, Singh N, Williams PM, Brown AP, Jenkins GJS (2009) Confounding experimental considerations in nanogenotoxicology. Mutagenesis 24:284–293
Gao G, Ze Y, Li B, Zhao X, Zhang T, Sheng L, Cheng J (2012) Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater 243:19–27
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconj chem 15(4):897–900
Griffitt RJ, Brown-Peterson NJ, Savin DA, Manning CS, Boube I, Ryan RA, Brouwer M (2012) Effects of chronic nanoparticulate silver exposure to adult and juvenile sheepshead minnows (Cyprinodon variegatus). Environ Toxicol Chem 31(1):160–167
Hsieh MS, Shiao NH, Chan WH (2009) Cytotoxic effects of CdSe quantum dots on maturation of mouse oocytes, fertilization, and fetal development. Int J Mol Sci 10(5):2122–2135
Jiang W, Kim BY, Rutka JT, Chan WC (2008) Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3(3):145–150
Juan H, XuYing W, Fei W, GuiFeng X, Zhen L, TianBao Z (2009) Effects of titanium dioxide nanoparticles on development and maturation of rat preantral follicle in vitro. Acad J Second Milit Med Univ 30(8):869–873
Kim HR, Park YJ, Shin DY, Oh SM, Chung KH (2013) Appropriate in vitro methods for genotoxicity testing of silver nanoparticles. Environ health toxicol 28:e2013003
Kosmehl T, Krebs F, Manz W, Erdinger L, Braunbeck T, Hollert H (2004) Comparative genotoxicity testing of rhine river sediment extracts using the comet assay with permanent fish cell lines (rtg-2 and rtl-w1) and the ames test. J Soil Sediment 4(2):84–94
Lankveld DPK, Oomen AG, Krystek P, Neigh A, Troost–de Jong AH, Noorlander CW, De Jong WH (2010) The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 31(32):8350–8361
Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, Kreyling WG (2010) Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials 31(25):6574–6581
Liu Z, Tabakman S, Welsher K, Dai H (2009) Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res 2(2):85–120
Menke AL, Spitsbergen JM, Wolterbeek AP, Woutersen RA (2011) Normal anatomy and histology of the adult zebrafish. Toxicol Pathol 39(5):759–775
Moor C, Lymberopoulou T, Dietrich VJ (2001) Determination of heavy metals in soils, sediments and geological materials by ICP-AES and ICP-MS. Mikrochim Acta 136(3–4):123–128
Nune SK, Gunda P, Thallapally PK, Lin YY, Laird Forrest M, Berkland CJ (2009) Nanoparticles for biomedical imaging. Expert Opin Drug Deliv 6(11):1175–1194
Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Jahnen-Dechent W (2007) Size-dependent cytotoxicity of gold nanoparticles. Small 3(11):1941–1949
Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Jahnen-Dechent W (2009) Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 5(18):2067–2076
Ramsden CS, Henry TB, Handy RD (2013) Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat Toxicol 126:404–413
Rathore M, Ray I, Mohanty NC, Maheshwari U, Dayal N, Suman R, Joshi DS (2014) Comparative in vivo assessment of sub-acute toxicity of gold and silver nanoparticles. J Nanopart Res 16:23–38
Schnurstein A, Braunbeck T (2001) Tail moment versus tail length—application of an in vitro version of the comet assay in biomonitoring for genotoxicity in native surface waters using primary hepatocytes and gill cells from zebrafish (Danio rerio). Ecotoxicol Environ Saf 49(2):187–196
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175(1):184–191
Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Doak SH (2009) NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30(23):3891–3914
Stelzer R, Hutz RJ (2009) Gold nanoparticles enter rat ovarian granulosa cells and subcellular organelles, and alter in vitro estrogen accumulation. J Reprod Dev 55(6):685–690
Strober, W. (2001). Trypan blue exclusion test of cell viability. Curr protoc immunol, A3–B
Tiedemann D, Taylor U, Rehbock C, Jakobi J, Klein S, Kues WA, Rath D (2014) Reprotoxicity of gold, silver, and gold–silver alloy nanoparticles on mammalian gametes. Analyst 139(5):931–942
Tsoli M, Kuhn H, Brandau W, Esche H, Schmid G (2005) Cellular uptake and toxicity of Au55 clusters. Small 1(8–9):841–844
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Wang C, Yu C (2013) Detection of chemical pollutants in water using gold nanoparticles as sensors: a review. Rev Anal Chem 32(1):1–14
Wang J, Zhu X, Zhang X, Zhao Z, Liu H, George R, Chen Y (2011) Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 83(4):461–467
Westerfield M, Institute of Neuroscience (1994) The zebrafish book: a guide for the laboratory use of zebrafish Danio (Brachydanio rerio). University of Oregon Press, Corvallis
Zhang XD, Guo ML, Wu HY, Sun YM, Ding YQ, Feng X, Zhang LA (2009) Irradiation stability and cytotoxicity of gold nanoparticles for radiotherapy. Int J Nanomed 4:165–173
Zhu RR, Wang SL, Chao J, Shi DL, Zhang R, Sun XY, Yao SD (2009) Bio-effects of nano-TiO2 on DNA and cellular ultrastructure with different polymorph and size. Mater Sci Eng C 29(3):691–696
Acknowledgments
The authors would like to thank MGM Institute of Health Sciences for providing the infrastructure. The authors thank Mr. Runit Patil, Laboratory Attendant for his efforts in maintenance of the zebrafish facility.
Authors’ contributions
Ms. Navami Dayal is a PhD student who conducted the experimental part comprising of synthesis and characterization of AuNPs, histopathology, and comet assay under the supervision of Dr. Mansee Thakur. Ms. Dayal also contribute to manuscript preparation. Dr. Dipry Singh conducted the electron microscopy analysis of zebrafish ovaries. Ms. Poonam Patil carried out all the dissection process of zebrafish and assisted Ms. Dayal in comet assay procedure. Comet assay procedure was conducted under the guidance and expertise of Dr. Geeta Vanage. Dr. D. S. Joshi contributes to conception of the research work and shares his expertise in nanotechnology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dayal, N., Thakur, M., Patil, P. et al. Histological and genotoxic evaluation of gold nanoparticles in ovarian cells of zebrafish (Danio rerio). J Nanopart Res 18, 291 (2016). https://doi.org/10.1007/s11051-016-3549-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3549-0