Abstract
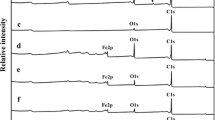
Mannosylation is a method commonly used to deliver nanomaterials to specific organs and tissues via cellular macrophage uptake. In this work, for the first time, we proposed a method that involves the binding of d-mannose to ethylenediamine to form mannosylated ethylenediamine, which is then coupled to oxidized and purified multiwalled carbon nanotubes. The advantage of this approach is that mannosylated ethylenediamine precipitates in methanol, which greatly facilitates the separation of this product in the synthesis process. Carbon nanotubes were oxidized using concentrated H2SO4 and HNO3 by conventional reflux method. However, during this oxidation process, carbon nanotubes generated carboxylated carbonaceous fragments (oxidation debris). These by-products were removed from the oxidized carbon nanotubes to ensure that the functionalization would occur only on the carbon nanotube surface. The coupling of mannosylated ethylenediamine to debris-free carbon nanotubes was accomplished using n-(3-dimethylaminopropyl)-n-ethylcarbodiimide and n-hydroxysuccinimide. Deconvoluted N1s spectra obtained from X-ray photoelectron spectroscopy gave binding energies of 399.8 and 401.7 eV, which we attributed to the amide and amine groups, respectively, of carbon nanotubes functionalized with mannosylated ethylenediamine. Deconvoluted O1s spectra showed a binding energy of 532.4 eV, which we suggest is caused by an overlap in the binding energies of the aliphatic CO groups of d-mannose and the O=C group of the amide bond. The functionalization degree was approximately 3.4 %, according to the thermogravimetric analysis. Scanning electron microscopy demonstrated that an extended carbon nanotube morphology was preserved following the oxidation, purification, and functionalization steps.








Similar content being viewed by others
References
Armentano I, Marinucci L, Dottori M, Balloni S, Fortunati E, Pennacchi M, Becchetti E, Locci P, Kenny JM (2011) Novel poly( L-lactide) PLLA/SWNTs nanocomposites for biomedical applications: material characterization and biocompatibility evaluation. J Biomater Sci Polym Ed 22:541–556
Beamson G, Briggs D (1992) High resolution XPS of organic polymers—The Scienta ESCA300 Database, Wiley Interscience, Appendices 3.1 and 3.2
Dai H (2002) Carbon nanotubes: synthesis, integration, and properties. Acc Chem Res 35:1035–1044
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, Kallitsis I, Galiotis C (2008) Chemical oxidation of multiwalled carbon nanotubes. Carbon 46:833–840
Elgrabli D, Dachraoui W, Menard-Moyon C, Liu XJ, Bégin D, Bégin-Colin S, Bianco A, Gazeau F, Alloyeau D (2015) Carbon nanotube degradation in macrophages: live nanoscale monitoring and understanding of biological pathway. ACS Nano 9:10113–10124
Figueiredo JF, Pereira MF (2010) The role of surface chemistry in catalysis with carbons. Catal Today 150:2–7
Gorityala BK, Ma J, Wang X, Chen P, Liu XW (2010) Carbohydrate functionalized carbon nanotubes and their applications. Chem Soc Rev 39:2925–2934
Jain SK, Gupta Y, Jain A, Saxena AR, Khare P, Jain A (2008) Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomed Nanotechnol 4:41–48
Jain AK, Dubey V, Mehra NK, Lodhi N, Nahar M, Mishra DK, Jain NK (2009) Carbohydrate-conjugated multiwalled carbon nanotubes: development and characterization. Nanomed Nanotechnol 5:432–442
Kam NWS, Dai H (2005) Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc 127:6021–6026
Kam NWS, O’Connell M, Wisdom JA, Dai HJ (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. PNAS 102:11600–11605
Kardosova A, Rosik J (1986) 13C NMR spectra of 2-O-β-glucopyranosylurono-D-manno-pyranose and 6-O-β-glucopyranosylurono-D-galactopyranose. Chem Papers 40:89–94
Lamberti M, Pedata P, Sannolo N, Porto S, Rosa A, Caraglia M (2015) Carbon nanotubes: properties, biomedical applications, advantages and risks in patients and occupationally-exposed workers. Int J Immunopathol Pharmacol 28:4–13
Lee SW, Kim BS, Chen S, Shao-Horn Y, Hammond PT (2009) Layer-by-layer assembly of all carbon nanotubes ultrathin films for electrochemical applications. J Am Chem Soc 131:671–679
Lin C, Lai PT, Liao SKS, Hung WT, Yang WB, Fang JM (2008) Using molecular iodine in direct oxidative condensation of aldoses with diamines: an improved synthesis of aldo-benzimidazoles and aldo-naphthimidazoles of carbohydrate analysis. J Org Chem 73:3848–3853
Lin C, Hung WT, Kuo CY, Liao KS, Liu YC, Yang WB (2010) I2-catalyzed oxidative condensation of aldoses with diamines: synthesis of aldo-naphthimidazoles for carbohydrate analysis. Molecules 15:1340–1353
Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H (2007) In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol 2:47–52
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68:6652–6660
Lockhoff O, Stadler P (1998) Syntheses of glycosylamides as glycolipid analogs. Carbohyd Res 314:13–24
Luo PG, Wang H, Gu L, Lu F, Lin Y, Christensen KA (2009) Selective interactions of sugar- functionalized single-walled carbon nanotubes with Bacillus spores. ACS Nano 3:3909–3916
Mitchell JP, Roberts KD, Langley J, Koentgen F, Lambert JN (1999) A direct method for the formation of peptide and carbohydrate dendrimers. Bioorg Med Chem Lett 9:2785–2788
Mitts E, Hixon RM (1944) The reaction of glucose with some amines. J Am Chem Soc 66:483–486
Murphy H, Papakonstantinou P, Okpalugo TIT (2006) Raman study of multiwalled carbon nanotubes functionalized with oxygen groups. J Vac Sci Technol B 24:715–720
Okpalugo TIT, Papakonstantinou P, Murphy H, McLaughlin J, Brown NMD (2005) High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 43:153–161
Popov VN (2004) Carbon nanotubes: properties and application. Mater Sci Eng R-Rep 43:61–102
Pruthi J, Mehra NK, Jain NK (2012) Macrophages targeting of amphotericin B through mannosylated multiwalled carbon nanotubes. J Drug Targ 20:593–604
Ramanathan T, Fisher FT, Ruoff RS, Brinson LC (2005) Amino-functionalized carbon for binding to polymers and biological system. Chem Mater 17:1290–1295
Rosca ID, Watari F, Uo M, Tsukasa A (2005) Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 43:3124–3131
Saetia K, Schnorr JM, Mannarino MM, Kim SY, Rutledge GC, Swager TM, Hammond PT (2014) Spray- layer-by-layer carbon nanotube/electrospun fiber electrodes for flexible chemiresistive sensor applications. Adv Funct Mater 24:492–502
Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Doyle CD, West JL, Billups WE, Ausman KD, Colvin VL (2006) Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 161:135–142
Stéfani D, Paula AJ, Vaz BG, Silva RA, Andrade NF, Justo GZ, Ferreira CV, Filho AGS, Eberlin MN, Alves OL (2011) Structural and proactive safety aspects of oxidation debris from multiwalled carbon nanotubes. J Hazard Mater 189:391–446
Verdejo R, Lamoriniere S, Cottam B, Bismarck A, Shaffer M (2007) Removal of oxidation debris from multi-walled carbon nanotubes. Chem Commun 5:513–515
Vieira RS, Oliveira MLM, Guibal E, Castollón ER, Beppu MM (2011) Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: an XPS investigation of mechanism. Coll Surf A 374:108–114
Volder MF, Tawfick SH, Baughman RH, Hart AJ (2013) Carbon nanotubes: present and future commercial applications. Science 339:535–539
Walker TE, London RE, Whaley TW, Barker R, Matwiyoff NA (1976) Carbon-13 nuclear magnetic resonance spectroscopy of [l-13C] enriched monosaccharides. signal assignments and orientational dependence of geminal and vicinal carbon-carbon and carbon-hydrogen spin–spin coupling constants. J Am Chem Soc 98:5807–5813
Wang W, Zhao X, Hu H, Chen D, Gu J, Deng Y, Sun J (2010) Galactosylated solid lipid nanoparticles with cucurbitacin B improves the liver targetability. Drug Deliv 17:114–122
Wu Z, Hamilton RF Jr, Wang Z, Holian A, Mitra S (2014) Oxidation debris in microwave functionalized carbon nanotubes: chemical and biological effects. Carbon 68:678–686
Xie S, Li W, Pan Z, Chang B, Sun L (2000) Mechanical and physical properties on carbon nanotube. J Phys Chem Solids 61:1153–1158
Zakaria AB, Picaud F, Rattier T, Pudle M, Saviot L, Chassagnon R, Lherminier J, Gharbi T, Micheau O, Herlem G (2015) Nanovectorization of TRAIL with single wall carbon nanotubes enhances tumor cell killing. Nano Lett 15:891–895
Acknowledgments
The authors gratefully acknowledge financial support from CNPq, FAPESP, INCT-Inomat, the Brazilian Nanotoxicology Network (Cigenanotox) and NanoBioss-SisNANO/MCTI. We also extend gratitude to the Nanostructured Materials Laboratory (XPS Facility) at LNNano/CNPEM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa, M., Martinez, D.S.T. & Alves, O.L. Alternative mannosylation method for nanomaterials: application to oxidized debris-free multiwalled carbon nanotubes. J Nanopart Res 18, 143 (2016). https://doi.org/10.1007/s11051-016-3399-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3399-9