Abstract
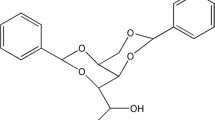
We report a method for tuning the nanoarchitectures of 1,3:2,4-di(3,4-dimethylbenzylidene) sorbitol (DMDBS) with poly(vinylidene fluoride) (PVDF) polymer matrices. Hydrophobic PVDF facilitated the formation of nanofibrils during heating. The self-assembly behaviors of DMDBS were further tuned by altering the different heat treatments. When the samples were prepared with a rapid heating rate (shorter annealing time), smaller amounts of melted PVDF were excluded due to the shorter time for aggregation of DMDBS, leading to larger complex structures of DMDBS and PVDF. Therefore, longer and thicker nanofibrils (around 100 nm) were observed using scanning electron microscopy. As the samples were prepared with a slow heating rate (longer annealing time), DMDBS had more time to aggregate, and therefore, larger amounts of melted PVDF were excluded. Smaller complex structures of DMDBS and PVDF caused the formation of shorter and thinner nanofibrils (around 40 nm). In addition, small-angle X-ray scattering results indicated that the longer and thicker nanofibrils were mostly excluded outside the PVDF crystalline bundles after cooling because they were too large to be easily incorporated between the PVDF crystalline lamellae. However, a large portion of the smaller and thinner nanofibrils was trapped between the crystalline lamellae after cooling due to their smaller sizes. As expected, the PVDF spherulitic morphologies were affected, but the PVDF crystalline microstructures were not significantly altered by the addition of DMDBS, as shown by the results from polarized optical microscopy and Fourier transform infrared spectroscopy.







Similar content being viewed by others
References
Basrur VR, Guo J, Wang C, Raghavan SR (2013) Synergistic gelation of silica nanoparticles and a sorbitol-based molecular gelator to yield highly-conductive free-standing gel electrolytes. ACS Appl Mater Interfaces 5:262–267
Boccaccio T, Bottino A, Capannelli G (2002) Characterization of PVDF membranes by vibrational spectroscopy. J Membr Sci 210:315–329
Bormashenko Y, Pogreb R, Stanevsky O (2004) Vibrational spectrum of PVDF and its interpretation. Polym Test 23:791–796
Chen W, Yang Y, Lee CH, Shen AQ (2008) Confinement effects on the self-assembly of 1, 3: 2, 4-di-p-methylbenzylidene sorbitol based organogel. Langmuir 24:10432–10436
Diehn KK, Oh H, Hashemipour R, Weiss RG, Raghavan SR (2014) Insights into organogelation and its kinetics from Hansen solubility parameters. Toward a prior predictions of molecular gelation. Soft Matter 10:2632–2640
Feng C, Khulbe KC, Matsuura T, Gopal R, Kaur S, Ramakrishn S, Khayet M (2008) Production of drinking water from saline water by air-gap membrane distillation using polyvinylidene fluoride nanofiber membrane. J Membr Sci 311:1–6
Kim GH, Hong SM, Seo Y (2009) Piezoelectric properties of poly(vinylidene fluoride) and carbon nanotube blends: β-phase development. Phys Chem Chem Phys 11:10506–10512
Kristiansen M, Werner M, Tervoort T, Smith P (2003) The binary system isotactic polypropylene/bis (3,4-dimethylbenzylidene) sorbitol: phase behavior, nucleation, and optical properties. Macromolecules 36:5150–5156
Lai W-C (2011) The effect of self-assembled nanofibrils on the morphology and microstructure of poly (l-lactic acid). Soft Matter 7:3844–3851
Lai W-C (2012) Microstructural investigation of concentric-circled poly(l-lactic acid) spherulites with self-assembled nanofibrils. RSC Adv 2:7221–7227
Lai W-C, Cheng L-T (2014) Preparation and characterization of novel poly(vinylidene fluoride) membranes using self-assembled dibenzylidene sorbitol for membrane distillation. Desalination 332:7–17
Lai W-C, Tseng S-J (2013) Tuning the self-assembled 1, 3: 2, 4-di (3, 4-dimethylbenzylidene) sorbitol nanoarchitectures using the phase inversion method. J Nanopart Res 15:2060
Lai W-C, Wu C-H (2010) Studies on the self-assembly of neat DBS and DBS/PPG organogels. J Appl Polym Sci 115:1113–1119
Lai W-C, Tseng S-C, Tung S-H, Huang Y-E, Raghavan SR (2009) Nanostructured polymers prepared using a self-assembled nanofibrillar scaffold as a reverse template. J Phys Chem B 113:8026–8030
Lipp J, Shuster M, Terry AE, Cohen Y (2006) Fibril formation of 1, 3: 2, 4-di (3, 4-dimethylbenzylidene) sorbitol in a polypropylene melt. Langmuir 22:6398–6402
Liu S, Yu W, Zhou C (2013) Solvents effects in the formation and viscoelasticity of DBS organogels. Soft Matter 9:864–874
Meng J, Yuan J, Kang Y, Zhang Y, Du Q (2011) Surface glycosylation of polysulfone membrane towards a novel complexing membrane for boron removal. J Colloid Interface Sci 368:197–207
Nahir TM, Qiu YJ, Williams JL (1994) Electrochemical characterization of a novel organic electrolyte gel. Electroanalysis 6:972–997
Neburchilov V, Martin J, Wang H, Zhang J (2007) A review of polymer electrolyte membranes for direct methanol fuel cells. J Power Sour 169:221–238
Schamper T, Jablon M, Randhawa MH, Senatore A, Warren JD (1986) Acid stable dibenzylidene sorbitol gelled clear solid antiperspirant formulations: I. J Soc Cosmet Chem 37:225–231
Shen H, Niu L, Fan K, Li J, Guan X (2014) Application of solubility parameters in 1, 3: 2, 4-bis (3, 4-dimethylbenzylidene) sorbitol organogel in binary organic mixtures. Langmuir 30:9176–9182
Smith TL, Masilamani D, Bui LK, Khanna YP, Bray RG, Hammond WB, Curran S, Belles J, Binder-Castelli S (1994) The mechanism of action of sugar acetals as nucleating agents for polypropylene. Macromolecules 27:3147–3155
Socrates G (1980) Infrared and Raman characteristic group: tables and charts. Wiley, New York
Wang K, Zhou CJ, Tang CY, Zhang Q, Du RN, Fu Q, Li L (2009) Rheologically determined negative influence of increasing nucleating agent content on the crystallization of isotactic polypropylene. Polymer 50:696–706
Watase M, Itagaki H (1998) Thermal and rheological properties of physical gels formed from benzylidene-d-sorbitol derivatives. Bull Chem Soc Jpn 71:1457–1466
Yan L, Li YS, Xiang CB (2005) Preparation of poly(vinylidene fluoride) (PVDF) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 46:7701–7706
Acknowledgments
We gratefully acknowledge the financial support from the Taiwan Ministry of Science and Technology (MOST 101-2628-E-032 -002 -MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, WC., Tseng, SJ. & Huang, PH. Self-assembled structures of 1,3:2,4-di(3,4-dimethylbenzylidene) sorbitol in hydrophobic polymer matrices prepared using different heat treatments. J Nanopart Res 17, 456 (2015). https://doi.org/10.1007/s11051-015-3267-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3267-z