Abstract
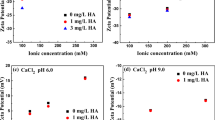
The transport behavior of titanium dioxide nanoparticles (TiO2 NPs, 30 nm in diameter) was studied in well-defined porous media composed of clean quartz sand over a range of solution chemistry under acidic conditions. Transport of TiO2 NPs was dramatically enhanced by humic substances (HS) at acidic pH (4.0, 5.0 and 6.0), even at a low HS concentration of 0.5 mg L−1. Facilitated transport of TiO2 NPs was likely attributable to the increased stability of TiO2 NPs and repulsive interaction between TiO2 NPs and quartz sands due to the adsorbed HS. The mobility of TiO2 NPs was also increased with increasing pH from 4.0 to 6.0. Although transport of TiO2 NPs was insensitive to low ionic strength, it was significantly inhibited by high concentrations of NaCl and CaCl2. In addition, calculated Derjaguin–Landau–Verwey–Overbeek (DLVO) interaction energy indicated that high energy barriers were responsible for the high mobility of TiO2 NPs, while the secondary energy minimum could play an important role in the retention of TiO2 NPs at 100 mmol L−1 NaCl. Straining and gravitational settlement of larger TiO2 NPs aggregates at 1 mg L−1 HS, pH 5.0, and 2 mmol L−1 CaCl2 could be responsible for the significant retention even in the presence of high energy barriers. Moreover, more favorable interaction between approaching TiO2 NPs and TiO2 NPs that had been already deposited on the collector resulted in a ripening-shape breakthrough curve at 2 mmol L−1 CaCl2. Overall, a combination of mechanisms including DLVO-type force, straining, and physical filtration was involved in the retention of TiO2 NPs over the range of solution chemistry examined in this study.





Similar content being viewed by others
References
Amirbahman A, Olson TM (1995) Deposition kinetics of humic matter-coated hematite in porous-media in the presence of Ca2+. Colloid Surf A 99:1–10. doi:10.1016/0927-7757(95)03134-Y
Bian SW, Mudunkotuwa IA, Rupasinghe T, Grassian VH (2011) Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 27:6059–6068. doi:10.1021/La200570n
Bouchard D, Zhang W, Chang XJ (2013) A rapid screening technique for estimating nanoparticle transport in porous media. Water Res 47:4086–4094. doi:10.1016/j.watres.2012.10.026
Bradford SA, Yates SR, Bettahar M, Simunek J (2002) Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resour Res 38: 63-1-63-12 doi: 10.1029/2002wr001340
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959. doi:10.1021/Cr0500535
Chen GX, Liu XY, Su CM (2011) Transport and retention of TiO2 rutile nanoparticles in saturated porous media under low-ionic-strength conditions: measurements and mechanisms. Langmuir 27:5393–5402. doi:10.1021/La200251v
Chen GX, Liu XY, Su CM (2012) Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ Sci Technol 46:7142–7150. doi:10.1021/Es204010g
Chowdhury I, Hong Y, Honda RJ, Walker SL (2011) Mechanisms of TiO2 nanoparticle transport in porous media: role of solution chemistry, nanoparticle concentration, and flowrate. J Colloid Interf Sci 360:548–555. doi:10.1016/j.jcis.2011.04.111
Cosgrove T (2005) Colloid science: principles, methods and applications. Blackwell Publishing, Oxford
Crittenden JC, Montgomery Watson Harza (Firm) (2005) Water treatment principles and design, 2nd edn. J. Wiley, Hoboken
Dietrich LAS, Sahu M, Biswas P, Fein JB (2012) Experimental study of TiO2 nanoparticle adhesion to silica and Fe(III) oxide-coated silica surfaces. Chem Geol 332:148–156. doi:10.1016/j.chemgeo.2012.09.043
Du WC, Sun YY, Ji R, Zhu JG, Wu JC, Guo HY (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monitor 13:822–828. doi:10.1039/C0em00611d
Elimelech M (1995) Particle deposition and aggregation: measurement, modelling, and simulation. Colloid and surface engineering series. Butterworth-Heinemann, Oxford
Espinasse B, Hotze EM, Wiesner MR (2007) Transport and retention of colloidal aggregates of C-60 in porous media: effects of organic macromolecules, ionic composition, and preparation method. Environ Sci Technol 41:7396–7402. doi:10.1021/Es0708767
Fang J, Shan XQ, Wen B, Lin JM, Owens G, Zhou SR (2011) Transport of copper as affected by titania nanoparticles in soil columns. Environ Pollut 159:1248–1256. doi:10.1016/j.envpol.2011.01.039
Fang J, Xu MJ, Wang DJ, Wen B, Han JY (2013) Modeling the transport of TiO2 nanoparticle aggregates in saturated and unsaturated granular media: effects of ionic strength and pH. Water Res 47:1399–1408. doi:10.1016/j.watres.2012.12.005
Ge YG, Schimel JP, Holden PA (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45:1659–1664. doi:10.1021/Es103040t
Godinez IG, Darnault CJG (2011) Aggregation and transport of nano-TiO2 in saturated porous media: effects of pH, surfactants and flow velocity. Water Res 45:839–851. doi:10.1016/j.watres.2010.09.013
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43:9216–9222. doi:10.1021/Es9015553
He YT, Wan JM, Tokunaga T (2008) Kinetic stability of hematite nanoparticles: the effect of particle sizes. J Nanopart Res 10:321–332. doi:10.1007/s11051-007-9255-1
He F, Zhang M, Qian TW, Zhao DY (2009) Transport of carboxymethyl cellulose stabilized iron nanoparticles in porous media: column experiments and modeling. J Colloid Interface Sci 334:96–102. doi:10.1016/j.jcis.2009.02.058
Jones EH, Su CM (2012) Fate and transport of elemental copper (Cu-0) nanoparticles through saturated porous media in the presence of organic materials. Water Res 46:2445–2456. doi:10.1016/j.watres.2012.02.022
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1–17. doi:10.1007/S11051-013-1692-4
Lin DH, Tian XL, Wu FC, Xing BS (2010) Fate and transport of engineered nanomaterials in the environment. J Environ Qual 39:1896–1908. doi:10.2134/Jeq2009.0423
Litton GM, Olson TM (1993) Colloid Deposition rates on silica bed media and artifacts related to collector surface preparation methods. Environ Sci Technol 27:185–193. doi:10.1021/Es00038a022
Loosli F, Le Coustumer P, Stoll S (2013) TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res 47:6052–6063. doi:10.1016/j.watres.2013.07.021
Lu YY, Xu XP, Yang K, Lin DH (2013) The effects of surfactants and solution chemistry on the transport of multiwalled carbon nanotubes in quartz sand-packed columns. Environ Pollut 182:269–277. doi:10.1016/j.envpol.2013.07.034
Mcdowellboyer LM, Hunt JR, Sitar N (1986) Particle-transport through porous-media. Water Resour Res 22:1901–1921. doi:10.1029/Wr022i013p01901
Menard A, Drobne D, Jemec A (2011) Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ Pollut 159:677–684. doi:10.1016/j.envpol.2010.11.027
Petosa AR, Brennan SJ, Rajput F, Tufenkji N (2012) Transport of two metal oxide nanoparticles in saturated granular porous media: role of water chemistry and particle coating. Water Res 46:1273–1285. doi:10.1016/j.watres.2011.12.033
Rahman T, George J, Shipley HJ (2013) Transport of aluminum oxide nanoparticles in saturated sand: effects of ionic strength, flow rate, and nanoparticle concentration. Sci Total Environ 463:565–571. doi:10.1016/j.scitotenv.2013.06.049
Saleh N, Kim HJ, Phenrat T, Matyjaszewski K, Tilton RD, Lowry GV (2008) Ionic strength and composition affect the mobility of surface-modified Fe-0 nanoparticles in water-saturated sand columns. Environ Sci Technol 42:3349–3355. doi:10.1021/Es071936b
Shih YH, Liu WS, Su YF (2012) Aggregation of stabilized TiO2 nanoparticle suspensions in the presence of inorganic ions. Environ Toxicol Chem 31:1693–1698. doi:10.1002/Etc.1898
Solovitch N, Labille J, Rose J, Chaurand P, Borschneck D, Wiesner MR, Bottero JY (2010) Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environ Sci Technol 44:4897–4902. doi:10.1021/Es1000819
Song L, Elimelech M (1993) Dynamics of colloid deposition in porous media: modeling the role of retained particles. Colloid Surf A 73:49–63. doi:10.1016/0927-7757(93)80006-Z
Tufenkji N, Elimelech M (2004) Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ Sci Technol 38:529–536. doi:10.1021/Es034049r
Wang Y et al (2012) Transport of titanium dioxide nanoparticles in saturated porous media under various solution chemistry conditions. J Nanopart Res 14:1095. doi:10.1007/S11051-012-1095-Y
Xu YL, Qin Y, Palchoudhury S, Bao YP (2011) Water-soluble iron oxide nanoparticles with high stability and selective surface functionality. Langmuir 27:8990–8997. doi:10.1021/La201652h
Yang WW et al (2014) TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate Tetrahymena thermophila. Environ Sci Technol 48:7568–7575. doi:10.1021/Es500694t
Yao KM, Habibian MM, Omelia CR (1971) Water and waste water filtration: concepts and applications. Environ Sci Technol 5:1105–1112. doi:10.1021/Es60058a005
Zhang Y, Chen YS, Westerhoff P, Crittenden J (2009) Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res 43:4249–4257. doi:10.1016/j.watres.2009.06.005
Zhou DM, Wang DJ, Cang L, Hao XZ, Chu LY (2011) Transport and re-entrainment of soil colloids in saturated packed column: effects of pH and ionic strength. J Soil Sediment 11:491–503. doi:10.1007/s11368-010-0331-2
Zhuang J, Qi J, Jin Y (2005) Retention and transport of amphiphilic colloids under unsaturated flow conditions: effect of particle size and surface property. Environ Sci Technol 39:7853–7859. doi:10.1021/Es050265j
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41171248, 41230858).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, R., Zhang, H., Tu, C. et al. Facilitated transport of titanium dioxide nanoparticles by humic substances in saturated porous media under acidic conditions. J Nanopart Res 17, 165 (2015). https://doi.org/10.1007/s11051-015-2972-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-2972-y