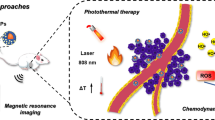
Abstract
Biocompatible nanoparticles (NPs) responding to the light, thermal, or magnetic excitation are attracting more attention for diagnosis and therapy of cancer. Design of an effective multifunctional complex based on those NPs is a key issue to be addressed, for example, integration of anti-tumor agents with nanoprobes has been considered as one of the successful strategies for combined cancer diagnosis and therapy. In this paper, we develop paclitaxel (PTX)-loaded PEGylation KMnF3 NP, with the size ranged from 18 to 23 nm, as MRI contrast agents for cancer imaging and drug delivery for chemotherapy. Preliminary cell tests demonstrated that PTX@PEG-KMnF3 NP is highly biocompatible. The NP has high loading capacity of PTX (0.7 mg PTX/mg Mn ions), enhanced solubility of PTX (0.16 mg PTX/ml vs 0.02 mg PTX/ml), and high releasing ratio (90 %) in the weak acid solution. As it was applied for in vivo imaging and therapy, the NP enhanced contrast of tumor’s MR images and PTX’s anti-tumor effect profoundly. The signal noise ratio of the cancer image increased 170 % as comparison to pre-injection with the injection dose of 1.15 mg Mn/kg. The drug delivery’s efficacy was also substantially improved, as the tumor growth inhibition effects reached 50 %, meanwhile only 30 % for pristine PTX. Our studies suggest that PTX-loaded KMnF3 NP might be useful as MR image-guided drug delivery for tumor treatment.







Similar content being viewed by others
References
Algar WR, Prasuhn DE, Stewart MH, Jennings TL, Blanco-Canosa JB, Dawson PE (2011) The controlled display of biomolecules on nanoparticles: a challenge suited to bioorthogonal chemistry. Bioconjug Chem 22:825–858
Ambruosi A, Yamamoto H, Kreuter J (2005) Body distribution of polysorbate-80 and doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after iv administration in rats. J Drug Target 13:535–542
Andersen A, Warren DJ, Brunsvig PF, Aamdal S, Kristensen GB, Olsen H (2006) High sensitivity assays for docetaxel and paclitaxel in plasma using solid-phase extraction and high-performance liquid chromatography with UV detection. BMC Clin Pharmacol 6:2–12
Belda-Iniesta C, de Castro Carpeno J, Casado Saenz E, Cejas Guerrero P, Perona R, Gonzalez Baron M (2006) Molecular biology of malignant gliomas. Clin Transl Oncol 8:635–641
Bozzola JJ, Russell LD (1992) Electron microscopy: principles and techniques for biologists. Jones and Bartlett Publishers, Boston, pp 16–148
Couvreur P, Vauthier C (2006) Nanotechnology: intelligent design to treat complex disease. Pharm Res 23:1417–1450
Datki Z, Juhász A, Gálfi M, Soós K, Papp R, Zádori D (2003) Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain Res Bull 62:223–229
Devanand VG, Ramasamy S, Ramakrishnan V, Kumar J (2013) Folate targeted PEGylated titanium dioxide nanoparticles as a nanocarrier for targeted paclitaxel drug delivery. Adv Powder Technol 24:947–954
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171
Gerwek LE (1998) Tumor pH: implications for treatment and novel drug design. Semin Radiat Oncol 8:176–182
Gregoriadis G (2007) Liposome technology: interactions of liposomes with the biological milieu. CRC Press, Boca Raton, pp 277–303
Greish K (2010) Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol 624:25–37
Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I (2005) NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer 92:1240–1246
Kim JH, Kim YS, Kim S, Park JH, Kim K, Choi K (2006) Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J Control Release 111:228–234
Liu ZJ, Song XX, Tang Q (2013) Development of PEGylated KMnF3 nanoparticles as a T1-weighted contrast agent: chemical synthesis, in vivo brain MR imaging, and accounting for high relaxivity. Nanoscale 5:5073–5079
Liu ZJ, Song XX, Xu XZ, Tang Q (2014) Biocompatible KMnF3 nanoparticular contrast agent with proper plasma retention time for in vivo magnetic resonance imaging. Nanotechnology 25:155101–155108
Lv PP, Ma YF, Yu R, Yue H, Ni DZ, Wei W (2012) Targeted delivery of insoluble cargo (paclitaxel) by PEGylated chitosan nanoparticles grafted with Arg-Gly-Asp (RGD). Mol Pharm 9:1736–1747
Maeda H, Bharate GY, Daruwalla J (2009) Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm 1:409–419
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46:6387–6392
Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R (2009) Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer–alendronate–taxane conjugate. Angew Chem Int Ed 48:2949–2954
Nie S, Xing Y, Kim GJ, Simons JW (2007) Nanotechnology applications in cancer. Annu Rev Biomed Eng 9:257–288
Ofir R, Seidman R, Rabinski T, Krup M, Yavelsky V, Weinstein Y (2002) Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ 9:636–642
Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC (1993) Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol 20:1–15
Siepmann F, Muschert S, Flament MP, Leterme P, Gayot A, Siepmann J (2006) Controlled drug release from gelucire-based matrix pellets: experiment and theory. Int J Pharm 317:136–143
Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS (2004) Chemotherapy of glioblastoma in rats using doxorubicin loaded nanoparticles. Int J Cancer 109:759–767
Tromsdorf UI, Bruns OT, Salmen SC, Beisiegel U, Weller H (2009) A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett 9:4434–4440
Wang Y, Xin D, Liu K, Zhu M, Xiang J (2009) Heparin-paclitaxel conjugates as drug delivery system: synthesis, self-assembly property, drug release, and antitumor activity. Bioconjug Chem 20:2214–2221
Yu MK, Jeong YY, Park J, Park S, Kim JW, Min JJ (2008) Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed 47:5362–5365
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (21361018), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2012BAE01B02), and the Fundamental Research Foundation by the Jiangxi Ministry of Education (GJJ14123). The authors acknowledge Jian-qi Li (Shanghai Key Lab of Magnetic Resonance), Xiao-zeng You (Nanjing University) for helpful discussion and in vivo MRI operation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Xx., Wan, Hp., Zhang, Js. et al. Paclitaxel-loaded KMnF3 nanoparticles for cancer imaging and therapy in vivo. J Nanopart Res 16, 2722 (2014). https://doi.org/10.1007/s11051-014-2722-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2722-6