Abstract
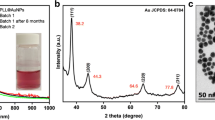
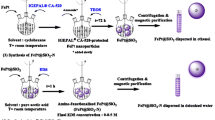
The high product yield of multi-branched core–shell Fe3−x O4@Au magnetic nanoparticles was synthesized used as magnetic separation platform and surface-enhanced Raman scattering (SERS) substrates. The multi-branched magnetic nanoparticles were prepared by a seed-mediated growth approach using magnetic gold nanospheres as the seeds and subsequent reduction of metal salt with ascorbic acid in the presence of a stabilizing agent chitosan biopolymer and silver ions. The anisotropic growth of nanoparticles was observed in the presence of chitosan polymer matrix resulting in multi-branched nanoparticles with a diameter over 100 nm, and silver ions also play a crucial role on the growth of multi-branched nanoparticles. We propose the mechanism of the formation of multi-branched nanoparticles while the properties of nanoparticles embedded in chitosan matrix are discussed. The surface morphology of nanoparticles was characterized with transmission electron microscopy, scanning electron microscopy, ultraviolet visible spectroscopy (UV–Vis), X-ray diffraction, and fourier transform infrared spectroscopy and 57Fe Mössbauer spectrometry. Additionally, the magnetic properties of the nanoparticles were also examined. We also demonstrated that the synthesized Fe3−x O4@Au multi-branched nanoparticle is capable of targeted separation of pathogens from matrix and sensing as SERS substrates.





Similar content being viewed by others
References
Aaron JS, Oh J, Larson TA, Kumar S, Milner TE, Sokolov KV (2006) Increased optical contrast in imaging of epidermal growth factor receptor using magnetically actuated hybrid gold/iron oxide nanoparticles. Opt Express 14:12930–12943. doi:10.1364/OE.14.012930
Brugnerotto J, Lizardi J, Goycoolea FM, Argüelles-Monal W, Desbrières J, Rinaudo M (2001) An infrared investigation in relation with chitin and chitosan characterization. Polymer 42:3569–3580. doi:10.1016/S0032-3861(00)00713-8
Carpenter EE, Kumbhar A, Wiemann JA, Srikanth H, Wiggins J, Zhou W, O’Connor CJ (2000) Synthesis and magnetic properties of gold–iron–gold nanocomposites. Mat Sci Eng A-Struct 286:81–86. doi:10.1016/S0921-5093(00)00681-X
Dai X, Fan Z, Lu Y, Ray PC (2013) Multifunctional nanoplatforms for targeted multidrug-resistant-bacteria theranostic applications. ACS Appl Mater Interfaces 5:11348–11354. doi:10.1021/am403567k
dos Santos DS, Goulet PJG, Pieczonka NPW, Oliveira ON, Aroca RF (2004) Gold nanoparticle embedded self-sustained chitosan films as substrates for surface-enhanced Raman scattering. Langmuir 20:10273–10277. doi:10.1021/la048328j
Guerrero-Martínez A, Barbosa S, Pastoriza-Santos I, Liz-Marzán LM (2011) Nanostars shine bright for you: Colloidal synthesis, properties and applications of branched metallic nanoparticles. Curr Opin Colloid Interface Sci 16:118–127. doi:10.1016/j.cocis.2010.12.007
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021. doi:10.1016/j.biomaterials.2004.10.012
Heitsch AT, Smith DK, Patel RN, Ress D, Korgel BA (2008) Multifunctional particles: magnetic nanocrystals and gold nanorods coated with fluorescent dye-doped silica shells. J Solid State Chem 181:1590–1599. doi:10.1016/j.jssc.2008.05.002
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int J Food Microbiol 71:235–244. doi:10.1016/S0168-1605(01)00609-2
Hong Y, Brown DG (2006) Cell surface acid–base properties of Escherichia coli and Bacillus brevis and variation as a function of growth phase, nitrogen source and C: N ratio. Colloids Surf B 50:112–119. doi:10.1016/j.colsurfb.2006.05.001
Jones MR, Osberg KD, Macfarlane RJ, Langille MR, Mirkin CA (2011) Templated Techniques for the Synthesis and Assembly of Plasmonic Nanostructures. Chem Rev 111:3736–3827. doi:10.1021/cr1004452
Jung G-B, Kim J-H, Burm JS, Park H-K (2013) Fabrication of chitosan-silver nanoparticle hybrid 3D porous structure as a SERS substrate for biomedical applications. Appl Surf Sci 273:179–183. doi:10.1016/j.apsusc.2013.02.010
Kawamura G, Yang Y, Fukuda K, Nogami M (2009) Shape control synthesis of multi-branched gold nanoparticles. Mater Chem Phys 115:229–234. doi:10.1016/j.matchemphys.2008.11.064
Kean T, Thanou M (2010) Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev 62:3–11. doi:10.1016/j.addr.2009.09.004
Larson TA, Bankson J, Aaron J, Sokolov K (2007) Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 18:325101
Liu M, Guyot-Sionnest P (2005) Mechanism of Silver(I)-assisted growth of gold nanorods and bipyramids. J Phys Chem B 109:22192–22200. doi:10.1021/jp054808n
Liu H, Du Y, Wang X, Sun L (2004) Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol 95:147–155. doi:10.1016/j.ijfoodmicro.2004.01.022
Mahmoud MA, El-Sayed MA (2012) Substrate effect on the plasmonic sensing ability of hollow nanoparticles of different shapes. J Phys Chem B 117:4468–4477. doi:10.1021/jp3085793
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Narayanan S, Sathy BN, Mony U, Koyakutty M, Nair SV, Menon D (2011) Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl Mater Interfaces 4:251–260. doi:10.1021/am201311c
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta 396:153–166. doi:10.1016/S0040-6031(02)00523-3
Potara M, Baia M, Farcau C, Astilean S (2012) Chitosan-coated anisotropic silver nanoparticles as a SERS substrate for single-molecule detection. Nanotechnology 23:055501. doi:10.1088/0957-4484/23/5/055501
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779. doi:10.1021/cr2001178
Salehizadeh H, Hekmatian E, Sadeghi M, Kennedy K (2012) Synthesis and characterization of core-shell Fe3O4-gold-chitosan nanostructure. J Nanobiotechnol 10:3
Sanvicens N, Pastells C, Pascual N, Marco MP (2009) Nanoparticle-based biosensors for detection of pathogenic bacteria. TrAC, Trends Anal Chem 28:1243–1252. doi:10.1016/j.trac.2009.08.002
Sapsford KE et al (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113:1904–2074. doi:10.1021/cr300143v
Sau TK, Rogach AL, Döblinger M, Feldmann J (2011) One-step high-yield aqueous synthesis of size-tunable multispiked gold nanoparticles. Small 7:2188–2194. doi:10.1002/smll.201100365
Schotter J, Bethge O, Maier T, Brueckl H (2008) Recognition of biomolecular interactions by plasmon resonance shifts in single- and multicomponent magnetic nanoparticles. Appl Phys Lett 93:144105–144105–144103 doi:10.1063/1.2992589
Song H-M, Wei Q, Ong QK, Wei A (2010) Plasmon-resonant nanoparticles and nanostars with magnetic cores: synthesis and magnetomotive imaging. ACS Nano 4:5163–5173. doi:10.1021/nn101202h
Tamer U, Gündoğdu Y, Boyacı İ, Pekmez K (2010) Synthesis of magnetic core–shell Fe3O4–Au nanoparticle for biomolecule immobilization and detection. J Nanopart Res 12:1187–1196. doi:10.1007/s11051-009-9749-0
Tamer U, Boyacı İ, Temur E, Zengin A, Dincer İ, Elerman Y (2011) Fabrication of magnetic gold nanorod particles for immunomagnetic separation and SERS application. J Nanopart Res 13:3167–3176. doi:10.1007/s11051-010-0213-y
Tamer U et al (2013) Gold–coated iron composite nanospheres targeted the detection of escherichia coli. Int J Mol Sci 14:6223–6240
Tram LLT, Cao C, Høgberg J, Wolff A, Bang DD (2012) Isolation and detection of Campylobacter jejuni from chicken fecal samples by immunomagnetic separation–PCR. Food Control 24:23–28. doi:10.1016/j.foodcont.2011.08.030
Wilson WW, Wade MM, Holman SC, Champlin FR (2001) Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods 43:153–164. doi:10.1016/S0167-7012(00)00224-4
Wu H-L, Chen C-H, Huang MH (2008) Seed-mediated synthesis of branched gold nanocrystals derived from the side growth of pentagonal bipyramids and the formation of gold nanostars. Chem Mater 21:110–114. doi:10.1021/cm802257e
Xu Z, Hou Y, Sun S (2007) Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J Am Chem Soc 129:8698–8699. doi:10.1021/ja073057v
Yang H, Qu L, Wimbrow AN, Jiang X, Sun Y (2007) Rapid detection of Listeria monocytogenes by nanoparticle-based immunomagnetic separation and real-time PCR. Int J Food Microbiol 118:132–138. doi:10.1016/j.ijfoodmicro.2007.06.019
Yuan H, Khoury CG, Hwang H, Wilson CM, Grant GA, Vo-Dinh T (2012) Gold nanostars: surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 23:075102
Zawadzki J, Kaczmarek H (2010) Thermal treatment of chitosan in various conditions. Carbohydr Polym 80:394–400. doi:10.1016/j.carbpol.2009.11.037
Acknowledgements
This work was supported by the Scientific and Technological Research Council (TUBITAK) grant numbers 108T794 and 111T983. We also acknowledge support from Gazi BAP, project number 05/2004-03 and 05/2011-69. We would like to thank Gokhan Demirel and Mehmet Yılmaz for 3D simulation. We also thank to Dr Saime Sebnem Çetin and Prof.Dr. Suleyman Ozcelik for XRD measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tamer, U., Onay, A., Ciftci, H. et al. High-yield aqueous synthesis of multi-branched iron oxide core–gold shell nanoparticles: SERS substrate for immobilization and magnetic separation of bacteria. J Nanopart Res 16, 2624 (2014). https://doi.org/10.1007/s11051-014-2624-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2624-7