Abstract
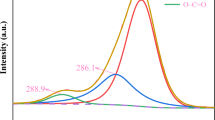
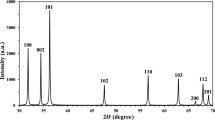
The influence of the hydrophilicity and length of the cation alkyl chain in imidazolium-based ionic liquids on the dispersability of ZnO nanoparticles by ultrasound treatment was studied by dynamic light scattering and advanced rheology. ZnO nanopowder synthesized by chemical vapor synthesis was used in parallel with one commercially available material. Before preparation of the dispersion, the nanoparticles characteristics were determined by transmission electron microscopy, X-ray diffraction, nitrogen adsorption with BET analysis, and FT-IR spectroscopy. Hydrophilic ionic liquids dispersed all studied nanopowders better and in the series of hydrophilic ionic liquids, an improvement of the dispersion quality with increasing length of the alkyl chain of the cation was observed. Especially, for ionic liquids with short alkyl chain, additional factors like nanoparticle concentration in the dispersion and the period of the ultrasonic treatment had significant influence on the dispersion quality. Additionally, nanopowder characteristics (crystallite shape and size as well as the agglomeration level) influenced the dispersion quality. The results indicate that the studied ionic liquids are promising candidates for absorber media at the end of the gas phase synthesis reactor allowing the direct preparation of non-agglomerated nanoparticle dispersions without supplementary addition of dispersants and stabilizers.













Similar content being viewed by others
References
Ali M, Winterer M (2010) ZnO nanocrystals: surprisingly ‘alive’. Chem Mater 22:85–91. doi:10.1021/cm902240c
Ali M, Friedenberger N, Spasova M, Winterer M (2009) A novel approach for chemical vapor synthesis of ZnO nanocrystals: optimization of yield, crystallinity. Chem Vap Depos 15:192–198. doi:10.1002/cvde.200806722
Becheri A, Dürr M, Lo Nostro P, Baglioni P (2008) Synthesis and characterization of zinc oxide nanoparticles: application to textile as UV-absorbers. J Nanopart Res 10:679–689. doi:10.1007/s11051-007-9318-3
Beek WJE, Wienk MM, Janssen RAJ (2004) Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv Mater 16:1009–1013. doi:10.1002/adma.200306659
Bonhôte P, Dias AP, Papagiorgiou N, Kalyanasundaram K, Grätzel M (1996) Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem 35:1168–1178
Brayner R, Ferrari-Illion R, Brivois N, Djediat S, Benedetti MF, Fievet F (2006) Toxilogical impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870. doi:10.1021/nl052326h
Buzzeo MC, Evans RG, Compton RG (2004) Non-haloaluminate room temperature ionic liquids in electrochemistry—a review. Chem Phys Chem 5:1106–1120. doi:10.1002/cphc.200301017
Chiappe C, Pieracini D (2005) Ionic liquids: solvent properties and organic reactivity. J Phys Org Chem 8:275–297. doi:10.1002/poc.836
Dupont J (2004) On the solid, liquid and solution structural organization of imidazolium ionic liquids. J Braz Chem Soc 15:341–350. doi:10.1590/S0103-50532004000300002
Dzyuba S, Bartsch RA (2002) Influence of structural variations in 1-alkyl(aralkyl)-3-methylimidazolium hexafluorophosphates and Bis(trifluoromethyl-sulfonyl)imides on physical properties of ionic liquids. Chem Phys Chem 3:161–166. doi:10.1002/1439-7641(20020215
Endres F, El Abedin SZ (2006) Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys 8:2101–2116. doi:10.1039/B600519P
Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, Wingett D (2008) Prefential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19:295103. doi:10.1088/0957-4484/19/29/295103
Hapiot P, Lagrost C (2008) Electrochemical reactivity in room temperature ionic liquids. Chem Rev 108:2238–2264. doi:10.1021/cr068686
Hossain MK, Ghosh SC, Boontongkong Y, Thanachayanant C, Dutta J (2005) Growth of zinc oxide nanowires and nanobelts for gas sensing applications. J Metastab Nanocryst Mater 23:27–30. doi:10.4028/www.scientific.net/JMNM.23.27
Huang Z, Zheng X, Yan D, Yin G, Liao X, Kang Y, Yao Y, Huang D, Hao B (2008) Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 24:4140–4144. doi:10.1021/la7035949
Kruis FE, Fissan H, Peled A (1998) Synthesis of nanoparticles in the gas phase for electronic, optical and magnetic applications—a review. J Aerosol Sci 29:511–535
Kubisa P (2009) Ionic liquids as solvents for polymerisation processes—progress and challenges. Prog Polym Sci 34:1333–1347. doi:10.1016/j.progpolymsci.2009.09.001
Lu J, Yan F, Texter J (2009) Advanced applications of ionic liquids in polymer science. Prog Polym Sci 34:431–448. doi:10.1016/j.progpolymsci.2008.12.001
Marsh KN, Deev A, Wu ACT, Tran E, Klamt A (2002) Room temperature ionic liquids as replacement for conventional solvents—a review, Korean. J Chem Eng 19:357–362
Ren X, Han D, Chen D, Tang F (2007) Lange-scale synthesis of hexagonal cone-shaped ZnO nanoparticles with a simple route and their application to photocatalytic degradation. Mater Res Bull 42:807–813. doi:10.1016/j.materresbull.2006.08.030
Schereen CW, Machado G, Dupont J, Fichtner PFP, Texeira SR (2003) Nanoscale Pt(O) particles prepared in imidazolium room temperature ionic liquids: synthesis from organometallic precursor, characterization and catalytic properties in hydrogenation reactions. Inorg Chem 42:4738–4743. doi:10.1021/ic034453r
Seddon KR (1997) Ionic liquids for clean technology. J Chem Technol Biotechnol 68:351–356
Senthilkumar K, Senthilkumar O, Yamauchi K, Sato M, Morito S, Ohba T, Nakamura M, Fujita Y (2009) Preparation of ZnO nanoparticles for bio-imaging applications. Phys Status Solidi B 246:885–888. doi:10.1002/pssb.200880606
Seow ZLS, Wang ASW, Thovasi V, Rose R, Ramakrishna S, Ho GW (2009) Controlled synthesis and application of ZnO nanoparticles, nanorods and nanospheres in dye-sensitized solar cells. Nanotechnology 20:045604. doi:10.1089/0957-4484/20/4/045604
Suslick KS, Price GJ (1999) Application of ultrasound to materials chemistry. Annu Rev Mater Sci 29:295. doi:10.1146/annurev.matsci.29.1.295
Swatloski RP, Holbrey JD, Rogers RD (2003) Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem 5:361–363. doi:10.1039/B4400A
Tani T, Mädler L, Pratsinis SE (2002) Homogenous ZnO nanoparticles by flame spray pyrolysis. J Nanopart Res 4:337–343. doi:10.1023/A:1021153419671
Tokuda H, Hayamizu K, Ishii K, Susan MABH, Watanabe M (2004) Physicochemical properties and structures of room temperature ionic liquids. 1. Variation of anionic species. J Phys Chem B 108:16593–16600. doi:10.1021/jp047480r
Tokuda H, Hayamizu K, Ishii K, Susan MABH, Watanabe M (2005) Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J Phys Chem B 109:6103–6110. doi:10.1021/jp044626d
Torimoto T, Tsuda T, Okazaki K, Kuwabata S (2010) New frontiers in materials science opened by ionic liquids. Adv Mater 22:1196–1221. doi:10.1002/adma.200902184
Ueno K, Watanabe M (2011) From colloidal stability in ionic liquids to advanced soft materials using unique media. Langmuir 27:9105–9115. doi:10.1021/la103942f
Wang L, Wang L, Ding W, Zhang F (2010) Acute toxicity of ferric oxide and zinc oxide nanoparticles in rats. J Nanosci Nanotechnol 10:8617–8624. doi:10.1166/jnn.2010.2483
Welton T (1999) Room temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99:2071–2083. doi:10.1021/cr980032t
Wittmar A, Ulbricht M (2012) Dispersions of various titania nanoparticles in two different ionic liquids. Ind Eng Chem Res 51:8425–8433. doi:10.1021/ie203010x
Wittmar A, Ruiz-Abad D, Ulbricht M (2012) Dispersion of silica nanoparticles in ionic liquids investigated with advanced rheology. J Nanopart Res 14:651–660. doi:10.1007/s11051-011-0651-1
Wittmar A, Gajda M, Gautam D, Dörfler U, Winterer M, Ulbricht M (2013) Influence of the cation alkyl chain length of imidazolium-based room temperature ionic liquids on the dispersibility of TiO2 nanopowders. J Nanopart Res 15:1463–1475. doi:10.1007/s11051-013-1463-2
Ye C, Liu W, Chen Y, Yu L (2001) Room-temperature ionic liquids: a novel versatile lubricant. Chem Commun 21:2244–2245. doi:10.1039/b106935g
Zhang H, Hong K, Mays JW (2002) Synthesis of block copolymers of styrene and methacrylate by conventional free radical polymerization in room temperature ionic liquids. Macromolecules 35:5738–5741. doi:10.1021/ma025518x
Zhao D, Wu M, Kou Y, Min E (2002) Ionic liquids: applications in catalysis. Catal Today 74:157–189
Acknowledgments
The financial support through the NanoEnergieTechnikZentrum (NETZ), an application-focused research project partially financed by the state of North Rhine-Westphalia and the European Union, is kindly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wittmar, A., Gautam, D., Schilling, C. et al. Stable zinc oxide nanoparticle dispersions in ionic liquids. J Nanopart Res 16, 2341 (2014). https://doi.org/10.1007/s11051-014-2341-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2341-2