Abstract
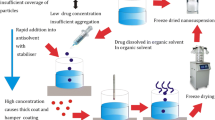
The purpose of the study was to prepare and characterize nanosuspensions that can maintain high and extended supersaturation to improve the dissolution and absorption of poorly soluble 10-hydroxycamptothecin (10-HCPT). 10-HCPT oral nanosuspensions (HCPT-Nanosuspensions) were produced on a laboratory-scale by microprecipitation- high pressure homogenization method. The particle morphology and the physical state were studied using transmission electron microscopy, X-ray powder diffraction (XRPD), and differential scanning calorimetry (DSC). Supersaturated dissolution tests were carried out with the paddle method. Caco-2 cell experiments were performed to imitate the oral absorption. The in vivo pharmacokinetics studies were undertaken in rats following oral administration. The 10-HCPT nanoparticles were 135 nm in dimension before lyophilization and were claviform or lump in shape. XRPD and DSC both confirmed that a portion of 10-HCPT was present in a crystalline state in nanosuspension. Supersaturated dissolution tests showed HCPT-Nanosuspensions could maintain high supersaturated level for an extended period time. The cell experiment on HCPT-Nanosuspensions showed a significantly higher uptake and greater membrane permeability compared with the other formulations. The pharmacokinetic test exhibited HCPT-Nanosuspensions had a similar pharmacokinetic performance with 10-HCPT solution. In conclusion, highly and extendedly supersaturated HCPT-Nanosuspensions have been prepared which could result in high peak concentration (C max) and great exposure (AUC) after oral administration.







Similar content being viewed by others
References
Bittner B, Guenzi A, Fullhardt P, Zuercher G, Gonzalez RC, Mountfield RJ (2002) Improvement of the bioavailability of colchicine in rats by co-administration of D-alpha-tocopherol polyethylene glycol 1000 succinate and a polyethoxylated derivative of 12-hydroxy-stearic acid. Arzneimittelforschung 52(9):684–688
Da Violante G, Zerrouk N, Richard I, Provot G, Chaumeil J, Arnaud P (2002) Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco-2/TC7 colon tumor cell cultures. Biol Pharm Bull 25(12):4
Gao J, Hugger ED, Beck-Westermeyer MS, Borchardt RT (2000) Esimating intestinal permeation of compounds using Caco-2 cell monolayers. Wiley, New York, p 3
Han R (1994) Highlight on the studies of anticancer drugs derived from plants in China. Stem Cells 12(1):53–63
Jacobs C, Müller RH (2002) Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res 19(2):189–194
Lenhardt T, Vergnault G, Grenier P, Scherer D, Langguth P (2008) Evaluation of nanosuspensions for absorption enhancement of poorly soluble drugs. In vitro transport studies across intestinal epithelial monolayers. AAPS J 10(3):435–438
Liversidge GG, Cundy KC (1995) Particle size reduction for improvement of oral bioavailability of hydrophobic drugs. I. Absolute oral bioavailability of nanocrystalline danazole in beagle dogs. Int J Pharm 125:91–97
Lu B, Zhang Z (2006) Novel colon-specific microspheres with highly dispersed hydroxycamptothecin cores: their preparation, release behavior, and therapeutic efficiency against colonic cancer. J Pharm Sci 95(12):2619–2630
Matteucci ME, Brettmann BK, Rogers TL, Elder EJ, Williams RO, I, Johnston KP (2007) Design of potent amorphous drug nanoparticles for rapid generation of highly supersaturated media. Mol Pharm 4(5):782–793
Matteucci ME, Paguio JC, Miller MA, Williams RO, III, Johnston KP (2008) Highly supersaturated solutions of amorphous drugs approaching predictions from configurational thermodynamic properties. J Phys Chem B 112(51):16675–16681
Matteucci ME, Paguio JC, Miller MA, Williams RO, III, Johnston KP (2009) Highly supersaturated solutions from dissolution of amorphous itraconazole microparticles at pH 6.8. Mol Pharm 6(2):375–385
Müller RH, Peters K, Becker R, Kruss B (1995) Nanosuspensions—a novel formulation for the i.v. administration of poorly soluble drugs. 7th Int Conf on Pharm Technol (APV/APGI), pp 491–492
Müller RH, Peters K, Becker R, Kruss B (1995) Nanosuspensions for the i.v. administration of poorly soluble drugs- stability during sterilization and long-term storage. 22nd Int Symp Control Release Bioact. Mater, Seattle.
Peters K, Leitzke S, Diederichs JE, Borner K, Hahn H, Müller RH, Ehlers S (2000) Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemother 45(1):77–83
Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G (2002) Eudragit RS10 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci 16:53–61
Prasad YV, Puthli SP, Eaimtrakarn S, Ishida M, Yoshikawa Y, Shibata N, Takada K (2003) Enhanced intestinal absorption of vancomycin with Labrasol and D-alpha-tocopheryl PEG 1000 succinate in rats. Int J Pharm 250(1):181–190
Pu X, Sun J, Li M, He Z (2009a) Formulation of nanosuspensions as a new approach for the delivery of poorly soluble drugs. Curr Nanosci 5(4):417–427
Pu X, Sun J, Wang Y, Wang Y, Liu X, Zhang P, Tang X, Pan W, Han J, He Z (2009b) Development of a chemically stable 10-hydroxycamptothecin nanosuspensions. Int J Pharm 379(1):167–173
Pu X, Sun J, Zhang P, Wang Y, Sun Y, He Z (2011) Study on pharmacokinetics of HCPT nanosuspensions with ability of inhibiting P-gp in rats after oral administration. China J Chinese Materia Medica 36(14):1959–1963
Sigfridsson K, Forssén S, Holländer P, Skantze U, de Verdier J (2007) A formulation comparison, using a solution and different nanosuspensions of a poorly soluble compound. Eur J Pharm Biopharm 67(2):540–547
Van Eerdenbrugh B, Froyen L, Martens JA, Blaton N, Augustijns P, Brewster M, Van den Mooter G (2007) Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze–dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int J Pharm 338(1–2):198–206
Wacher VJ, Wong S, Wong HT (2002) Peppermint oil enhances cyclosporine oral bioavailability in rats: comparison with D-alpha-tocopheryl poly(ethylene glycol 1000) succinate (TPGS) and ketoconazole. J Pharm Sci 91(1):77–90
Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA (1966) Plant antitumor agent. 1. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acumianta. J Am Chem Soc 88(16):3888–3890
Collnot E, Baldes C, Schaefer U, Edgar K, Wempe M, Lehr CM (2010) Vitamin E TPGS P-glycoprotein inhibition mechanism influence: on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol Pharm 7(3):10
Zhang R, Cai Q, Lindsey J, Li Y, Chambless B, Naguib F (1997) Antitumor activity and pharmacokinetics following oral administration of natural product DNA topoisomerase I inhibitors 10-hydroxycamptothecin and camptothecin in SCID mice bearing human breast cancer xenografts. Int J Oncol 10(6):1147–1156
Zhang L, Sun M, Guo R, Jiang Z, Liu Y, Jiang X, Yang C (2006) Chitosan surface-modified hydroxycamptothecin loaded nanoparticles with enhanced transport across Caco-2 cell monolayer. J Nanosci Nanotechnol 6(9):2912–2920
Zhang Z, Tan S, Feng SS (2012) Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 33(19):4889–4906
Acknowledgments
The authors would like to acknowledge the support of this study by the National Natural Science Foundation of China (NSFC), No. 81173008 and the National Basic Research Program of China (973 program, 2009CB930300).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pu, X., Sun, J., Han, J. et al. Nanosuspensions of 10-hydroxycamptothecin that can maintain high and extended supersaturation to enhance oral absorption: preparation, characterization and in vitro/in vivo evaluation. J Nanopart Res 15, 2043 (2013). https://doi.org/10.1007/s11051-013-2043-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2043-1