Abstract
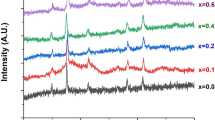
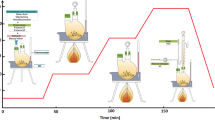
A facile solvothermal approach was used to synthesize stable, superparamagnetic manganese ferrite nanoparticles with relatively small sizes (<10 nm) and enhanced magnetic features. Tetraethylene glycol has been used in all the syntheses as a biocompatible and stabilizing agent. By varying the oxidation state of manganese precursor, Mn(acac)2 to Mn(acac)3, different sizes, 8 and 5 nm, of MnFe2O4 nanoparticles were obtained respectively, while by tailoring the synthetic conditions iron-rich Mn0.77Fe2.23O4 nanoparticles have been isolated with identical sizes and enhanced saturation magnetization. The magnetization values increased from 58.2 to 68.3 Am2/kg and from 53.3 to 60.2 Am2/kg for the nanoparticles of 8 and 5 nm, respectively. Blocking temperature (T B), ranging from 80 to 180 K, and anisotropy constant (K eff), ranging from 1.5 × 105 to 4.9 × 105 J/m3, were found higher for the iron-rich samples and associated with size and composition effects.







Similar content being viewed by others
References
Adireddy S, Lin C et al (2009) Size-controlled synthesis of quasi-monodisperse transition-metal ferrite nanocrystals in fatty alcohol solutions. J Phys Chem C 49:20800–20811
Antic B, Kremenovic A et al (2012) Magnetization enhancement and cation valences in nonstoichiometric (MnFe)3−δ nanoparticles. J Appl Phys 111:6–074309
Broese Van Groenou A et al (1968) Magnetism, microstructure and crystak chemistry of spinel ferrites. Mater Sci Eng 3:317–392
Cao H, Wang G et al (2006) Shape and magnetic properties of single-crystalline hematite (α-Fe2O3) nanocrystals. ChemPhysChem 7:1897–1901
Carta D, Casula MF et al (2009) A structural and magnetic investigation of the inversion degree in ferrite nanocrystals. J Phys Chem C 113:8606–8615
Charles RG, Pawlikowski MA (1958) Comparative heat stabilities of some metal acetylacetonate chelates. J Phys Chem 62:440–444
Chen J, Sorensen C et al (1996) Size-dependent magnetic properties of MnFe2O4 fine particles synthesized by coprecipitation. Phys Rev B 54:9288–9296
Colombo M, Carregal-Romero S et al (2012) Biological applications of magnetic nanoparticles. Chem Soc Rev 41:4306–4334
Cornell RM, Schwertmann U (2000) The iron oxides: structure, properties, reactions, occurrence and uses. Annu Rev Mater Sci 30:545–610
Garcia-Otero J, Porto M, Rivas J, Bunde A (2000) Influence of dipolar interaction on magnetic properties of ultrafine ferromagnetic particles. Phys Rev Lett 84:167–170
Gillot B, Laarj M, Kacim S (1997) Reactivity towards oxygen and cation distribution of manganese iron spinel Mn3−x Fe x O4 (0 ≤ x ≤ 3) fine powders studied by thermogravimetry and IR spectroscopy. J Mater Chem 7:827–831
Hergt R, Dutz S, Müller R, Zeisberger M (2006) Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. J Phys: Condens Matter 18:2919–2934
Hu H, Tian Z et al (2011) Surfactant-controlled morphology and magnetic property of manganese ferrite nanocrystal contrast agent. Nanotechnology 22:7–085707
Jang J, Nah H et al (2012) Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew Chem Int Ed 48:1234–1238
Jiles D (1991) Introduction to magnetism and magnetic materials. Chapman & Hall.CRC press LLC, Florida
Jun Y, Lee J, Cheon J (2008) Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew Chem Int Ed 47:5122–5135
Karakoti AS, Das S, Thevuthasan S, Seal S (2011) PEGylated inorganic nanoparticles. Angew Chem Int Ed 50:1980–1994
Kim D, Zeng H et al (2009) T1 and T2 relaxivities of succimer-coated MFe2O4 (M = Mn2+, Fe2+ and Co2+) inverse spinel ferrites for potential use as phase-contrast agents in medical MRI. J Magn Magn Mater 32:3899–3904
Kneller EF, Luborsky FE (1963) Particle-size dependence of coercivity and remanence of single domain particles. J Appl Phys 34:656–658
Kodama RH (1999) Magnetic nanoparticles. J Magn Magn Mater 200:359–372
Köseoglua Y, Alana F, Tana M, Yilginb R, Öztürkb M (2012) Low temperature hydrothermal synthesis and characterization of Mn doped cobalt ferrite nanoparticles. Ceram Int 38:3625–3634
Krishnan KM (2010) Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics and therapy. IEEE Trans Magn 46:2523–2558
Kumar P (2010) Magnetic behavior of surface nanostructured 50 nm nickel thin films. Nanoscale Res Lett 5:1596–1602
Kwon SG, Piao Y et al (2007) Kinetics of monodisperse iron oxide nanocrystal formation by “heating-up” process. J Am Chem Soc 129:12571–12584
Li X (2011) Single-crystalline α-Fe2O3 oblique nanoparallelepipeds: High-yield synthesis, growth mechanism and structure enhanced gas-sensing properties. Nanoscale 3:718–724
Li H, Wu H, Xiao G (2010) Effects of synthetic conditions on particle size and magnetic properties of NiFe2O4. Powder Technol 198:157–166
Lima E, Biasi E et al (2010) Surface effects in the magnetic properties of crystalline 3 nm ferrite nanoparticles chemically synthesized. J Appl Phys 108:10–103919
Liu C, Zou B et al (2000) Chemical control of superparamagnetic properties of magnesium and cobalt spinel ferrite nanoparticles through atomic level magnetic couplings. J Am Chem Soc 122:6263–6267
Lu A, Salabas E, Schuth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Néel L (1948) Magnetic properties of ferrites: ferrimagnetism and antiferromagnetism. Ann Phys 3:137–165
Neuberger T, Schopf B, Hofmann H et al (2005) Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater 293:483–496
Nlebedim IC, Snyder JE et al (2012) Anisotropy and magnetostriction in non-stoichiometric cobalt ferrite. IEEE Trans Magn 48:3084–3087
Pereira C, Pereira AM et al (2012) Superparamagnetic MFe2O4 (M = Fe, Co, Mn) nanoparticles: tuning the particle size and magnetic properties through a novel one-step coprecipitation route. Chem Mater 24:1496–1504
Rondinone AJ, Liu C et al (2001) Determination of magnetic anisotropy distribution and anisotropy constant of manganese spinel ferrite nanoparticles. J Phys Chem B 105:7967–7971
Salafranca J, Gazquez J et al (2012) Surfactant organic molecules restore magnetism in metal-oxide nanoparticle surfaces. Nano Lett 12:2499–2503
Schmid G (2010) Nanoparticles: from theory to application. Wiley-VCH, Weinheim
Shim JH, Lee S, Min BI (2007) Abnormal spin structure of manganese ferrite investigated by 57Fe NMR. Phys Rev B 75:5–134406
Sickafus K, Wills J, Grimes N (1999) Structure of spinel. J Am Ceram Soc 82:3279–3292
Spaldin N (2003) Magnetic materials: fundamentals and device applications. Cambridge University Press, Cambridge
Vargas J, Srivastava A et al (2011) Tuning the thermal relaxation of transition-metal ferrite nanoparticles through their intrinsic magnetocrystalline anisotropy. J Appl Phys 110:064304–064306
Verwey E, Heilmann E (1947) Physical properties and cation arrangements of oxides with spinel structures I. Cation arrangement in spinels. J Chem Phys 15:174–180
Vestal CR, Zhang ZJ (2003) Effects of surface coordination chemistry on the magnetic properties of MnFe2O4 spinel ferrite nanoparticles. J Am Chem Soc 125:9828–9833
Waldron RD (1955) Infrared spectra of ferrites. Phys Rev 99:1727–1735
Walton RI (2002) Subcritical solvothermal synthesis of condensed inorganic materials. Chem Soc Rev 31:230–238
Xu B, Wang X (2012) Solvothermal synthesis of monodisperse nanocrystals. Dalton Trans 41:4719–4725
Yang H, Zhang C et al (2010) Water-soluble superparamagnetic manganese ferrite nanoparticles for magnetic resonance imaging. Biomaterials 31:3667–3673
Acknowledgments
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Thales. Investing in knowledge society through the European Social Fund. The authors would like to thank the Assoc. Prof. C. Lioutas for his great assistance in TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vamvakidis, K., Sakellari, D., Angelakeris, M. et al. Size and compositionally controlled manganese ferrite nanoparticles with enhanced magnetization. J Nanopart Res 15, 1743 (2013). https://doi.org/10.1007/s11051-013-1743-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1743-x