Abstract
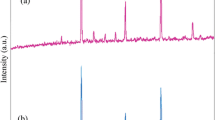
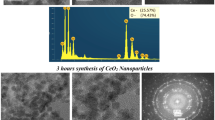
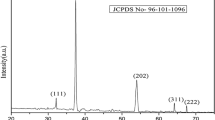
Physicochemical analysis on the precipitate samples of the cationic cetyltrimethylammonium bromide (CTAB) adsorbed onto nanocube CaCO3 particles (NcCP) in aqueous ammonia rich (NH4 +) solution was initially examined. The amount of CTAB added to the (<100 nm) NcCP ranging from 0.04 to 88.5 mM was prepared under room temperature aqueous alkaline condition and characterized by thermogravimetry/differential thermogravimetric analysis (TGA/DTA), Raman spectroscopy (RS), scanning electron microscopy, transmission electron microscopy (TEM), gas chromatograph combined with mass spectrometer analysis (GC–MS), and powder X-ray diffraction pattern. RS, GC–MS, and TGA/DTA analyses indicate that only layer of CTAB molecules were present on the surface of the NcCP. Moreover, this thin sheet layer was morphologically observed by the TEM image (particularly at 88 mM concentration of CTAB). In general, adsorption of CTAB molecules onto NcCP under aqueous alkaline medium had no effect on the cubic crystal structure and particle morphology. The present study confirms the adsorption mechanism of cationic surfactant onto NcCP colloids model and contributes to the better understanding of the possible structural arrangement of the sorbed surfactant molecules onto the NcCP-aqueous alkaline interface by simple characterization method. This investigation is expected to create new, low-cost route to produce promising nanopowders and conversion to hollow particles with multi-component porous surface shell wall.






Similar content being viewed by others
References
Beach S, Newsted J, Coady K, Giesy J (2006) Ecotoxicological evaluation of perfluorooctanesulfonate (PFOS). In: Albert LA, Voogt P, Gerba CP et al (eds) Reviews of environmental contamination and toxicology, vol 186 reviews of environmental contamination and toxicology. Springer, New York, pp 133–174. doi:10.1007/0-387-32883-1_5
Bjorklund RB, Arwin H, Järnström L (1994) Adsorption of anionic and cationic polymers on porous and non-porous calcium carbonate surfaces. Appl Surf Sci 75(1–4):197–203. doi:10.1016/0169-4332(94)90159-7
Blandamer MJ, Cullis PM, Soldi LG, Chowdoji Rao K, Subha MCS (1996) Effects of added dotab on the <i>cmc</i> and enthalpy of micelle formation at 298.2 K for CTAB(aq). J Therm Anal Calorim 46(6):1583–1588. doi:10.1007/bf01980764
Butler MF, Frith WJ, Rawlins C, Weaver AC, Heppenstall-Butler M (2008) Hollow calcium carbonate microsphere formation in the presence of biopolymers and additives. Cryst Growth Des 9(1):534–545. doi:10.1021/cg8008333
Bu-Yao Z, Yu-Jun G, Hong-Jian W, Guo-Xi Z (1996) An investigation on the micelle-catalytic hydrolysis and ammonolysis of carboxylic acid esters. Acta Phys Chim Sin 12(02):109–113. doi:10.3866/PKU.WHXB19960204
Cooke DJ, Gray RJ, Sand KK, Stipp SLS, Elliott JA (2010) Interaction of ethanol and water with the \(10\bar{1}4\) surface of calcite. Langmuir 26(18):14520–14529. doi:10.1021/la100670k
Cui ZG, Shi KZ, Cui YZ, Binks BP (2008) Double phase inversion of emulsions stabilized by a mixture of CaCO3 nanoparticles and sodium dodecyl sulphate. Colloids Surf Physicochem Eng Aspects 329(1–2):67–74. doi:10.1016/j.colsurfa.2008.06.049
Cui ZG, Yang LL, Cui YZ, Binks BP (2009) Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant. Langmuir 26(7):4717–4724. doi:10.1021/la903589e
Cui ZG, Cui YZ, Cui CF, Chen Z, Binks BP (2010) Aqueous foams stabilized by in situ surface activation of CaCO3 nanoparticles via adsorption of anionic surfactant. Langmuir 26(15):12567–12574. doi:10.1021/la1016559
De Klerk WP (1996) Thermal Analysis of some propellants and explosives with DSC and TG/DTA. http://handle.dtic.mil/100.2/ADA320678. Accessed Dec 1996
Dendramis AL, Schwinn EW, Sperline RP (1983) A surface-enhanced Raman scattering study of CTAB adsorption on copper. Surf Sci 134(3):675–688. doi:10.1016/0039-6028(83)90065-1
Etzler FM, Conners JJ (1991) A DSC/TGA method for determination of the heat of vaporization. Thermochim Acta 189(2):185–192. doi:10.1016/0040-6031(91)87114-c
Fuji M, Takai C, Tarutani Y, Takei T, Takahashi M (2007) Surface properties of nanosize hollow silica particles on the molecular level. Adv Powder Technol 18:81–91. doi:10.1163/156855207779768124
Fuji M, Shin T, Watanabe H, Takei T (2011) Shape-controlled hollow silica nanoparticles synthesized by an inorganic particle template method. Adv Powder Technol. doi:10.1016/j.apt.2011.06.002
Goworek J, Kierys A, Gac W, Borówka A, Kusak R (2009) Thermal degradation of CTAB in as-synthesized MCM-41. J Therm Anal Calorim 96(2):375–382. doi:10.1007/s10973-008-9055-6
Guo X, Deng Y, Gu D, Che R, Zhao D (2009) Synthesis and microwave absorption of uniform hematite nanoparticles and their core-shell mesoporous silica nanocomposites. J Mater Chem 19(37):6706–6712. doi:10.1039/B910606e
Gurses A, Yalcin M, Sozbilir M, Dogar C (2003) The investigation of adsorption thermodynamics and mechanism of a cationic surfactant, CTAB, onto powdered active carbon. Fuel Process Technol 81(1):57–66. doi:10.1016/s0378-3820(03)00002-x
Han YS, Hadiko G, Fuji M, Takahashi M (2005) A novel approach to synthesize hollow calcium carbonate particles. Chem Lett 34(2):152–153. doi:10.1246/Cl.2005.152
Howard SC, Craig VSJ (2009) Adsorption of the cationic surfactant cetyltrimethylammonium bromide to silica in the presence of sodium salicylate: surface excess and kinetics. Langmuir 25(22):13015–13024. doi:10.1021/la901889m
Ivanova NI, Shchukin ED (1993) Mixed adsorption of ionic and non-ionic surfactants on calcium carbonate. Colloids Surf Physicochem Eng Aspects 76:109–113. doi:10.1016/0927-7757(93)80068-p
Jiang J, Liu J, Liu C, Zhang G, Gong X, Liu J (2011) Roles of oleic acid during micropore dispersing preparation of nano-calcium carbonate particles. Appl Surf Sci 257(16):7047–7053. doi:10.1016/j.apsusc.2011.03.001
Li W, Han YC, Zhang JL, Wang BG (2005) Effect of ethanol on the aggregation properties of cetyltrimethylammonium bromide surfactant. Colloid J 67(2):159–163. doi:10.1007/s10595-005-0075-7
Liu L-G, Mernagh TP (1990) Phase transitions and Raman spectra of calcite at high pressures and room temperature. Am Mineral 75(7–8):801–806
Martinez-Ramirez S, Sanchez-Cortes S, Garcia-Ramos JV, Domingo C, Fortes C, Blanco-Varela MT (2003) Micro-Raman spectroscopy applied to depth profiles of carbonates formed in lime mortar. Cem Concr Res 33(12):2063–2068. doi:10.1016/s0008-8846(03)00227-8
Myers D (1999) Surfaces, interfaces, and colloids: principles and applications. Wiley, New York
Nassrallah-Aboukaïs N, Boughriet A, Laureyns J, Aboukaïs A, Fischer JC, Langelin HR, Wartel M (1998) Transformation of vaterite into cubic calcite in the presence of copper(ii) species. Chem Mater 10(1):238–243. doi:10.1021/cm970405r
Ninness BJ (2001) A molecular investigation of absorption onto mineral pigments. Dissertation, The University of Maine, Maine
Osman MA, Suter UW (2002) Surface treatment of calcite with fatty acids: structure and properties of the organic monolayer. Chem Mater 14(10):4408–4415. doi:10.1021/cm021222u
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid-water interface. Adv Colloid Interface Sci 110(3):75–95. doi:10.1016/j.cis.2004.03.001
Sand KK, Yang M, Makovicky E, Cooke DJ, Hassenkam T, Bechgaard K, Stipp SLS (2010) Binding of ethanol on calcite: the role of the OH bond and its relevance to biomineralization. Langmuir 26(19):15239–15247. doi:10.1021/la101136j
Sharma KP, Kumaraswamy G, Ly I, Mondain-Monval O (2009) Self-assembly of silica particles in a nonionic surfactant hexagonal mesophase. J Phys Chem B 113(11):3423–3430. doi:10.1021/jp810769g
Sharma KP, Aswal VK, Kumaraswamy G (2010) Adsorption of nonionic surfactant on silica nanoparticles: structure and resultant interparticle interactions. J Phys Chem B 114(34):10986–10994. doi:10.1021/jp1033799
Shi X, Rosa R, Lazzeri A (2010) On the coating of precipitated calcium carbonate with stearic acid in aqueous medium. Langmuir 26(11):8474–8482. doi:10.1021/la904914h
Stepkowska ET, Sulek Z, Perez-Rodriguez JL, Justo A, Maqueda C (1991) Thermal and microstructural studies on mud with additives. J Therm Anal Calorim 37(7):1497–1511. doi:10.1007/bf01913483
Stodghill SP, Smith AE, O’Haver JH (2004) Thermodynamics of micellization and adsorption of three alkyltrimethylammonium bromides using isothermal titration calorimetry. Langmuir 20(26):11387–11392. doi:10.1021/la047954d
Sun S, Birke RL, Lombardi JR (1990) Surface-enhanced Raman spectroscopy of surfactants on silver electrodes. J Phys Chem 94(5):2005–2010. doi:10.1021/j100368a052
Swami A, Kumar A, Sastry M (2003) Formation of water-dispersible gold nanoparticles using a technique based on surface-bound interdigitated bilayers. Langmuir 19(4):1168–1172. doi:10.1021/la026523x
Takai C, Fuji M, Fujimoto K (2011) Skeletal silica nanoparticles prepared by control of reaction polarity. Chem Lett 40(12):1346–1348. doi:10.1246/Cl.2011.1346
Tyrode E, Rutland MW, Bain CD (2008) Adsorption of CTAB on hydrophilic silica studied by linear and nonlinear optical spectroscopy. J Am Chem Soc 130(51):17434–17445. doi:10.1021/ja805169z
Virtudazo RVR, Watanabe H, Fuji M, Takahashi M (2010) A simple approach to form hydrothermally stable templated hollow silica nanoparticles. Characterization and control of interfaces for high quality advanced materials, vol III. Wiley, New York. doi:10.1002/9780470917145.ch14
Wald SA, Winding CC (1971) Differential thermal analysis using high frequency dielectric heating I. Theory and equipment. Polym Eng Sci 11(1):57–63. doi:10.1002/pen.760110110
Wang C, Sheng Y, Zhao X, Pan Y, Hari B, Wang Z (2006) Synthesis of hydrophobic CaCO3 nanoparticles. Mater Lett 60(6):854–857. doi:10.1016/j.matlet.2005.10.035
Wang C, Liu Y, Bala H, Pan Y, Zhao J, Zhao X, Wang Z (2007) Facile preparation of CaCO3 nanoparticles with self-dispersing properties in the presence of dodecyl dimethyl betaine. Colloids Surf Physicochem Eng Aspects 297(1–3):179–182. doi:10.1016/j.colsurfa.2006.10.045
White SN (2009) Laser Raman spectroscopy as a technique for identification of seafloor hydrothermal and cold seep minerals. Chem Geol 259(3–4):240–252. doi:10.1016/j.chemgeo.2008.11.008
Williams CT, Yang Y, Bain CD (2000) Total internal reflection sum-frequency spectroscopy: a strategy for studying molecular adsorption on metal surfaces. Langmuir 16(5):2343–2350. doi:10.1021/la991009l
Wu C (1994) Laser light scattering determination of the surfactant interface thickness of spherical polystyrene microlatices. Macromolecules 27(24):7099–7102. doi:10.1021/ma00102a015
Yamasaki N, Tang W, Ke J (1992) Low-temperature sintering of calcium carbonate by a hydrothermal hot-pressing technique. J Mater Sci Lett 11(13):934–936. doi:10.1007/bf00729099
Zhao F, Du Y-K, Xu J-K, Liu S-F (2006) Determination of surfactant molecular volume by atomic force microscopy. Colloid J 68(6):784–787. doi:10.1134/s1061933x06060172
Zhao L, Feng J, Wang Z (2009) Synthesis and modification of calcium carbonate nanoparticles via a bobbling method. Sci China Ser B Chem 52(7):924–929. doi:10.1007/s11426-009-0125-9
Acknowledgments
The authors gratefully acknowledge that this research was partially supported by the Japanese Government Ministry of Education, Culture, Sports, Science and Technology (MEXT; Monbukagakusho Scholarship) and Grant-in-Aid for Scientific Research 22310066 (2010-2012). We are also thankful to C.C. Chua-Nakar (Reviewer-Inorganic Chemistry, Ramon Sison Review Center) for the useful technical discussion and assistance with this study.
Conflict of interest
The authors declares that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Virtudazo, R.V.R., Fuji, M., Takai, C. et al. Characterization on the precipitate sample of cetyltrimethylammonium bromide adsorbed onto nanocube CaCO3 particles from aqueous-ammonia-rich solution. J Nanopart Res 14, 1304 (2012). https://doi.org/10.1007/s11051-012-1304-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1304-8