Abstract
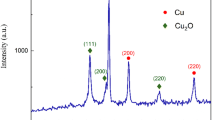
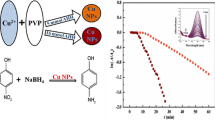
Copper nanoparticles are prepared and size characterized using FESEM and HRTEM. Poly ethylene glycol (PEG), carboxy methyl cellulose (CMC) and poly N vinyl pyrrolidone (PVP) are used as the nanoparticle stabilizers. The reduction reactions of fourteen differently aromatic ring substituted nitro benzenes are carried out with NaBH4 solution catalyzed by the copper nanoparticles with PEG, CMC and PVP as stabilizers. Using time variance UV absorbance spectra, the progress of the reactions are kinetically monitored, the parameters such as product % yield, time of reaction and rate coefficient values are determined and used for deducing the comparative catalytic efficiency. The copper nanoparticles as synthesized using biopolymers such as PEG and CMC produce better reaction parameters than the PVP stabilized Cu nps. Also the effect of substituents in the aromatic ring of the nitro compounds reveals that −I substituents containing nitro compounds are better catalyzed than the +I substituents. The sizes of the three Cu nps are found to be 6, 12 and 15 nm corresponding to PEG, CMC and PVP stabilizers respectively.










Similar content being viewed by others
References
Arul Dhas N, Paul Raj C, Gedanken A (1998) Synthesis, characterization, and properties of metallic copper nanoparticles. Chem Mater 10:1446–1452
Bai X, Gao Y, Liu H, Zheng L (2009) Synthesis of amphiphilic ionic liquids terminated gold nanorods and their superior catalytic activity for the reduction of nitro compounds. J Phys Chem C 113:17730–17736
Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC (2011) Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. 2: 015009. doi:10.1088/2043-6262/2/1/015009
David MD, Somnath B, Ya W, Merlin LB (2009) Nanoparticle-containing membranes for the catalytic reduction of nitroaromatic compounds. Langmuir 25:1865–1871
Dinda E, Rashid MdH, Biswas M, Mandal TK (2010) Redox-active ionic-liquid-assisted one-step general method for preparing gold nanoparticle thin films: applications in refractive index sensing and catalysis. Langmuir 26:17568–17580
Gamble AB, Garner J, Gordon CP, Conner O, Sean MJ, Keller PA (2007) Aryl nitro reduction with iron powder or stannous chloride under ultrasonic irradiation. Synth Commun 37:2777–2786
Ghosh SK, Mandal M, Kundu S, Nath S, Pal T (2004) Bimetallic Pt–Ni nanoparticles can catalyze reduction of aromatic nitro compounds by sodium borohydride in aqueous solution. Appl Catal A 268:61–66
Isabel P-S, Luis ML-M (2002) Formation of PVP-protected metal nanoparticles in DMF. Langmuir 18:2888–2894
Ivan P, Mirela F-L, Sonja M, Goranka L, Ivica C, Mladen L (2007) Rapid, efficient and selective reduction of aromatic nitro compounds with sodium borohydride and Raney nickel. J Mol Catal A 274:202–207
Kapoor S, Gopinathan C (1998) Reduction and aggregation of silver, copper and cadmium ions in aqueous solutions of gelatin and carboxymethyl cellulose. Radiat Phys Chem 53:165–170
Kapoor S, Palit DK, Mukherjee T (2002) Preparation, characterization and surface modification of Cu metal nanoparticles. Chem Phys Lett 355:383–387
Kyoko K, Tamao I, Masatake H (2009) Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J Mol Catal A 298:7–11
Lakshmi Kantam M, Rahman A, Bandyopadhyay T, Mahender Reddy N, Choudary BM (1998) Reduction of nitroaromatics with a new heterogenised MCM–silylamine palladium (II) catalyst. J Mol Catal A 133:293–295
Le Bars J, Specht U, Bradley JS, Blackmond DG (1999) A catalytic probe of the surface of colloidal palladium particles using heck coupling reactions. Langmuir 15:7621–7625
Li Y, Boone E, El-Sayed MA (2002) Size effects of PVP-Pd nanoparticles on the catalytic suzuki reactions in aqueous solution. Langmuir 18:4921–4925
Lisiecki I, Pileni MP (1993) Synthesis of copper metallic clusters using reverse micelles as microreactors. J Am Chem Soc 115:3887–3896
Liu Z, Yang Y, Liang J, Zhaokang H, Li S, Peng S, Qian Y (2003) Synthesis of copper nanowires via a complex-surfactant-assisted hydrothermal reduction process. J Phys Chem B 107:12658–12661
Mikihiro T, Yukihiro M, Kenji H, Yoon S-H, Mochida I, Nagashima H (2008) Chemoselective hydrogenation of nitroarenes with carbonnanofiber-supported platinum and palladium nanoparticles. Org Lett 10:1601–1604
Mohapatra S, Kumar RK, Maji TK (2011) Green synthesis of catalytic and ferromagnetic gold nanoparticles. Chem Phys Lett 508:76–79
Moshfegh AZ (2009) Nanoparticle catalysts. J Phys D 42:233001
Mott D, Galkowski J, Wang L, Luo J, Zhong C-J (2007) Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23:5740–5745
Narayanan R, El-Sayed MA (2003) Effect of catalysis on the stability of metallic nanoparticles: suzuki reaction catalyzed by pvp-palladium nanoparticles. J Am Chem Soc 125:8340–8347
Nurettin S, Hava O, Ozgur O, Nahit A (2010) A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols. Appl Catal B 101:137–143
Park BK, Jeong S, Kim D, Moon J, Lim S, Kim JS (2007) Synthesis and size control of monodisperse copper nanoparticles by polyol method. J Colloid Interface Sci 311:417–424
Pradhan N, Pal A, Pal T (2001) Catalytic reduction of aromatic nitro compounds by coinage metal nanoparticles. Langmuir 17:1800–1802
Rahman A, Jonnalagadda SB (2008) Swift and selective reduction of nitroaromatics to aromatic amines with Ni-boride-silica catalysts system at low temperature. Catal Lett 123:264–268
Saha A, Ranu B (2008) Highly chemoselective reduction of aromatic nitro compounds by copper nanoparticles ammonium formate. J Org Chem 73:6867–6870
Salavati-Niasari M, Davar F, Mir N (2008) Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Polyhedron 27:3514–3518
Toshima N, Yonezawa T (1998) Bimetallic nanoparticlesÈnovel materials for chemical and physical applications. New J Chem 22:1179–1201
Weiyong Y, Wang Y, Liu H, Zheng W (1996) Preparation and characterization of polymer-protected Pt/Co bimetallic colloids and their catalytic properties in the selective hydrogenation of cinnamaldehyde. J Mol Catal A 112:113
Wu S-H, Chen D-H (2004) Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J Colloid Interface Sci 273:165–169
Wunder S, Polzer F, Yan L, Mei Y, Ballauff M (2010) Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J Phys Chem C 114:8814–8820
Xin-dong M, Evans DG, Kou Y (2004) A general method for preparation of PVP-stabilized noble metal nanoparticles in room temperature ionic liquids. Catal Lett 97:151–154
Zhou M, Wang B, Rozynek Z, Xie Z, Fossum JO, Xiaofeng Y, Raaen S (2009) Minute synthesis of extremely stable gold nanoparticles. Nanotechnology 20:505606
Acknowledgments
The authors thank NCNSNT, University of Madras for the FE-SEM and HR-TEM results. L. P thanks for URF of the University of Madras.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santhanalakshmi, J., Parimala, L. The copper nanoparticles catalysed reduction of substituted nitrobenzenes: effect of nanoparticle stabilizers. J Nanopart Res 14, 1090 (2012). https://doi.org/10.1007/s11051-012-1090-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1090-3