Abstract
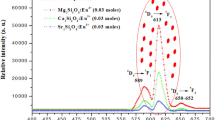
This study explores the viability of rare earth-doped zirconia nanophosphors as probable candidates for white light emission. Undoped ZrO2 and single- and double-doped ZrO2:M (where M = Tb3+ and Eu3+) nanophosphors have been synthesized using a simple sonochemical process. The products were characterized using X-ray diffraction, scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), energy dispersive X-ray spectroscopy (EDS), and photoluminescence spectrophotometry. The SEM micrographs show that resultant nanoparticles have dendritic shape. TEM and HRTEM studies showed that the size of the majority of the nanoparticles were around 28 ± 5 nm. Characteristic blue and green emission from Tb3+ ions and red from Eu3+ dopant ions were observed. The CIE coordinates of the double-doped ZrO2:Tb3+ (1.2 %):Eu3+ (0.8 %) nanophosphor lie in the white light region of the chromaticity diagram and show promise as good phosphor materials for new lighting devices.











Similar content being viewed by others
References
Agger JR, Anderson MW, Pemble ME, Terasaki O, Nozue Y (1998) Growth of quantum-confined indium phosphide inside MCM-41. J Phys Chem B 102:3345–3353
Assefa Z, Haire RG, Raison PE (2004) Photoluminescence and Raman studies of Sm3+ and Nd3+ ions in zirconia matrices: example of energy transfer and host–guest interactions. Spectrochim Acta A 60(1–2):89–95
Bhargava RN, Gallaghar D, Hong X, Nurmikko A (1994) Optical properties of manganese-doped nanocrystals of ZnS. Phys Rev Lett 72:416–419
Blasse G (1966) On the Eu3+ fluorescence of mixed metal oxides. IV. The photoluminescent efficiency of Eu3+ activated oxides. J Chem Phys 45(7):2356–2360
Bohe AE, Gamboa JA, Pasquevich JM, Tolley AJ, Pelegrina JL (2000) Microstructural characterization of ZrO2 particles prepared by reaction of gaseous ZrCl4 with Fe2O3. J Am Ceram Soc 83:755–760. doi:10.1111/j.1151-2916.2000.tb01270.x
Cao J, Qiu X, Luo B, Liang Y, Zhang Y, Tan R, Zhao M, Zhu Q (2004) Synthesis and room-temperature ultraviolet photoluminescence properties of zirconia nanowires. Adv Funct Mater 14(3):243–246
Chen L, Liu Y, Li Y (2004) Preparation and characterization of ZrO2:Eu3+ phosphors. J Alloys Compd 381(1–2):266
De la Rosa E, Diaz-Torres LA, Salas P, Rodrıguez RA (2005) Visible light emission under UV and IR excitation of rare earth doped ZrO2 nanophosphor. Opt Mater 27(7):1320–1325
De la Rosa-Cruz E, Diaz-Torres LA, Salas P, Rodrıguez RA, Kumar GA, Meneses MA, Mosino JF, Hernandez JM, Barbosa-Garcia O (2003) Luminescent properties and energy transfer in ZrO2:Sm3+ nanocrystals. J Appl Phys 94(5):3509
Diaz-Torres LA, De la Rosa E, Salas P, Romero VH, Angeles-Chavez C (2008) Efficient photoluminescence of Dy3+ at low concentrations in nanocrystalline ZrO2. J Solid State Chem 181(1):75–80
Fouassier C (1997) Luminescent materials. Curr Opin Solid State Mater Sci 2(2):231–235
Fujishiro F, Mochizuki S (2009) Reversible photo-induced spectral change and defect creation in ZrO2. Phys Stat Sol C 6(1):354–357
Garvie RC, Goss MF (1986) Intrinsic size dependence of the phase transformation temperature in zirconia microcrystals. J Mater Sci 21(4):1253–1257
Ghosh R, Patra A (2006) Role of surface coating in ZrO2/Eu3+ nanocrystals. Langmuir 22(14):6321–6327
Ghosh R, Priolkar KR, Patra A (2007) Understanding the local structures of Eu and Zr in Eu2O3 doped and coated ZrO2 nanocrystals by EXAFS study. J Phys Chem C 111(2):571–578
Gu F, Wang SF, Lu MK, Zhou GJ, Liu SW, Xu D, Yuan DR (2003) Effect of Dy3+ doping and calcination on the luminescence of ZrO2 nanoparticles. Chem Phys Lett 380(1–2):185–189
Hoefdraad HE (1975) The charge-transfer absorption band of Eu3+ in oxides. J Solid State Chem 15(2):175–177
Hunt RWG (1991) Measuring colors. Applied science & industrial technology series, vol 60. Ellis Horwood, New York
Kemler J (1969) Luminescent screens: photometry and colorimetry. Iliffe, London
Kumari L, Li WZ, Xu JM, Leblanc RM, Wang DZ, Li Y, Guo H, Zhang J (2009) Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst Growth Des 9(9):3874–3880
Lai L-J, Lu H-C, Chen H-K, Cheng B-M, Lin M-I, Chu T-C (2005) Photoluminescence of zirconia films with VUV excitation. J Electron Spectrosc Relat Phenom 144–147:865
Liang J, Deng Z, Jiang X, Li F, Li Y (2002) Photoluminescence of tetragonal ZrO2 nanoparticles synthesized by microwave irradiation. Inorg Chem 41(14):3602–3604
Mondal A, Ram S (2004) Monolithic t-ZrO2 nanopowder through a ZrO(OH)2·xH2O polymer precursor. J Am Ceram Soc 87(12):2187–2194
Mondal A, Ram S (2008) Enhanced phase stability and photoluminescence of Eu3+ modified t-ZrO2 nanoparticles. J Am Ceram Soc 91(1):329–332
Moona BK, Kwona M, Jeonga JH, Kimb C-S, Yic S-S, Kimd PS, Choid H, Kim JH (2007) Synthesis and luminescence characteristics of Eu3+-doped ZrO2 nanoparticles. J Lumin 122–123:855–857
Navio JA, Hidalgo MC, Colon G, Bottta SG, Litter MI (2001) Preparation and physicochemical properties of ZrO2 and Fe/ZrO2 prepared by a sol−gel technique. Langmuir 17(1):202–210
Nelson JA, Brant EL, Wagner MJ (2003) Nanocrystalline Y2O3:Eu phosphors prepared by alkalide reduction. Chem Mater 15(3):688–693
Patra A, Friend CS, Kapoor R, Prasad PN (2003) Effect of crystal nature on upconversion luminescence in Er3+:ZrO2 nanocrystals. Appl Phys Lett 83:284–286. doi:10.1063/1.1592891
Purohit RD, Saha S, Tyagi AK (2006) Combustion synthesis of nanocrystalline ZrO2 powder: XRD, Raman spectroscopy and TEM studies. Mater Sci Eng B 130(1–3):57–60
Reisfeld R, Saraidarov T, Pietraszkiewicz M, Lis S (2001) Luminescence of europium(III) compounds in zirconia xerogels. Chem Phys Lett 349(3–4):266–270
Romero VH, De la Rosa E, López-Luke T, Salas P, Angeles-Chavez C (2010) Brilliant blue, green and orange–red emission band on Tm3+-, Tb3+- and Eu3+-doped ZrO2 nanocrystals. J Phys D 43(46):465105
Salas P, Angeles C, Montoya JA, De la Rosa E, Dıaz-Torres LA, Martınez A, Romero-Romo MA, Morales J (2005) Synthesis, characterization and luminescence properties of ZrO2:Yb3+–Er3+ nanophosphor. Opt Mater 27(7):1295–1300
Shukla S, Seal S (2004) Thermodynamic tetragonal phase stability in sol−gel derived nanodomains of pure zirconia. J Phys Chem B 108(11):3395–3399
Somiya S, Akiva J (1999) Hydrothermal zirconia powders: a bibliography. J Eur Ceram Soc 19(1):81–87
Speghini A, Bettinelli M, Riello P, Bucella S, Benedetti A (2005) Preparation, structural characterization, and luminescence properties of Eu3+-doped nanocrystalline ZrO2. J Mater Res 20(10):2780–2791
Sundar Manoharan S, Rao M (2004) Sonochemical synthesis of nano materials. In: Nalwa HS (ed) Encyclopedia of nanoscience and nanotechnology. American Scientific Publishers, Los Angeles, pp 67–82
Suslick KS (1990) Sonochemistry. Science 247:1439
Trave A, Buda F, Fasolino A (1996) Band-gap engineering by III–V infill in sodalite. Phys Rev Lett 77:5405–5408
Wang YQ, Yin LX, Palhcik O, Hacohen YR, Koltypin Y, Gedanken A (2001) Sonochemical synthesis of layered and hexagonal yttrium−zirconium oxides. Chem Mater 13(4):1248–1251
Yashima M, Ohtake K, Kakihana M, Arashi H, Yoshimura M (1996) Determination of the tetragonal cubic phase boundary of Zr1−x R x O2−x/2 (R = Nd, Sm, Y, Er, Yb) by Raman scattering. J Phys Chem Solids 57:17–24
Zhang H, Fu X, Niu S, Xin Q (2008) Blue emission of ZrO2:Tm nanocrystals with different crystal structure under UV excitation. J Non-Cryst Solids 354(14):1559–1563
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, S., Dutta, D.P., Manoj, N. et al. Sonochemically synthesized rare earth double-doped zirconia nanoparticles: probable candidate for white light emission. J Nanopart Res 14, 814 (2012). https://doi.org/10.1007/s11051-012-0814-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0814-8