Abstract
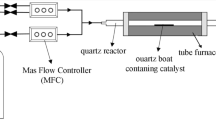
Worm-shaped carbon nanofibers (WCNFs) were synthesized in bulk by chemical vapour deposition at 680 °C using iron carboxylate as catalyst precursors and sodium chloride as catalyst support. The products were characterized by scanning electron microscopy, transmission electron microscopy, Raman spectroscopy and X-ray diffraction method. The purity of the purified products was determined by thermal analysis. The WCNF yield was 6700% relative to catalyst. The simplicity, environmental friendliness and use of easily available low-cost precursors are the advantage of this synthesis technique.







Similar content being viewed by others
References
Bahome MC, Jewell LL, Padayachy K, Hildebrandt D, Glasser D, Datye AK, Coville NJ (2005) Fischer–Tropsch synthesis over iron catalysts supported on carbon nanotubes. Appl Catal A 287:60–67
Brataas A (2008) Nanoelectronics: spin surprise in carbon. Nature 452:419–420
Chen XQ, Zhang SL, Dikin DA, Ding WQ, Ruoff RS, Pan LJ (2003) Mechanics of carbon nanocoils. Nano Lett 3:1299–1304
Cheng J, Zou X, Zhang H, Li F, Ren P, Zhu G, Su Y, Wang M (2008) Growth of Y-shaped carbon nanofibers from ethanol flames. Nanoscale Res Lett 3:295–298
Demicheva OV, Meshkov GB, Sinitsyna OV, Tomishko AG, Yaminsky IV (2008) Multiwall carbon nanotube tips for scanning probe microscopy. Nanotechnol Russ 3:704–709
Dillon AC, Jones KM, Bekkedahl TA, Kiang CH, Bethune DS, Heben MJ (1997) Storage of hydrogen in single-walled carbon nanotubes. Nature 386:377–379
Douglas RK, Alexander S (2010) Graphene versus carbon nanotubes for chemical sensor and fuel cell applications. Analyst 135:2790–2797
Edwards AB, Garner CD, Roberts KJ (1997) In situ QXAFS study of the pyrolytic decomposition of nickel formate dihydrate. J Phys Chem B 101:20–26
Furuya Y, Hashishin T, Iwanaga H, Motojima S, Hishikawa Y (2004) Interaction of hydrogen with carbon coils at low temperature. Carbon 42:331–335
Iijima S (1991) Helical microtubules of graphitic. Carbon 354:56–58
Jan E, Hendricks JL, Husaini L, Burns SMR, Sereno A, Martin DC, Kotov NA (2009) Layered carbon nanotube-polyelectrolyte electrodes outperform traditional neural interface materials. Nano Lett 9:4012–4018
Jesus JCD, Gonzalez I, Quevedo A, Puerta T (2005) Thermal decomposition of nickel acetate tetrahydrate: an integrated study by TGA, QMS and XPS techniques. J Mol Catal A: Chem 288:283–291
Kathyayini H, Reddy KV, Nagy JB, Nagaraju N (2008) Synthesis of carbon nanotubes over transition metal ions supported on Al(OH)3. Indian J Chem (Sec B) 47A:663
Milne WI, Teo KBK, Amaratunga GAJ, Legagneux P, Gangloff L, Schnell JP, Semet V, Thien VB, Groening O (2004) Carbon nanotubes as field emission sources. J Mater Chem 14:933–943
Panchakarla LS, Govindaraj A (2007) Carbon nanostructures and graphite-coated metal nanostructures obtained by pyrolysis of ruthenocene and ruthenocene–ferrocene mixtures. Bull Mater Sci 30:23
Qi X, Qin C, Zhong W, Au C, Ye X, Du Y (2010a) Large-scale synthesis of carbon NanoMaterials by catalytic chemical vapor deposition: a review of the effects of synthesis parameters and magnetic properties. Materials 3:4142–4174
Qi X, Zhong W, Deng Y, Au C, Du Y (2010b) Synthesis of helical carbon nanotubes, worm-like carbon nanotubes and nanocoils at 450 °C and their magnetic properties. Carbon 48:365–376
Schwarz JA, Contescu C, Contescu A (1995) Methods for preparation of catalytic materials. Chem Rev 95:477–510
Volodin A, Buntinx D, Ahlskog M, Fonseca A, Nagy JB, Haesendonck CV (2004) Coiled carbon nanotubes as self-sensing mechanical resonators. Nano Lett 4:1775–1779
Wang Y, Shi Z, Yin J (2010) Unzipped multiwalled carbon nanotubes for mechanical reinforcement of polymer composites. J Phys Chem C 114:19621
Yoshimura K, Nakano K, Miyake T, Hishikawa Y, Motojima S (2006) Effectiveness of carbon microcoils as a reinforcing material for a polymer matrix. Carbon 44:2833–2838
Acknowledgments
The authors would like to thank Defense Research and Development Organization (DRDO), Government of India for financial assistance and also to SAIF, IITB and central instrumental facilities, CECRI for providing analytical services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravindra, R., Bhat, B.R. Synthesis of worm-shaped carbon nanofibers over a sodium chloride support. J Nanopart Res 14, 656 (2012). https://doi.org/10.1007/s11051-011-0656-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-011-0656-9