Abstract
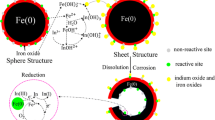
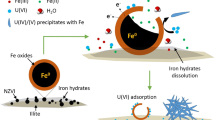
Zero-valent iron nanoparticles are effective remediators of uranium from solution. It is postulated that the improved core crystallinity and the migration of impurity phases to the nanoparticle surfaces induced by annealing may improve their corrosion resistance and reactive lifespan. The ability of annealed and non-annealed Fe and FeNi nanoparticles to remediate a U-contaminated effluent from AWE, Aldermaston was investigated. Nanoparticles (of diameter typically between 0 and 100 nm) were introduced to the effluent and allowed to react for 7 days during which the liquid and nanoparticulate solids were periodically sampled. In all the systems, the maximum U-uptake occurred within 1 h of introduction, with variable efficiency. The Fe nanoparticles removed 98% of the total U from solution, resulting in a final U-concentration of <4 μg/L. A rapid release of Fe into solution was recorded early in the reaction period: attributed to limited partial dissolution of the nanoparticles. Annealing the Fe nanoparticles did not affect their efficiency but the dissolution of Fe was significantly reduced and X-ray Photoelectron Spectroscopy indicated slower progressive oxidation. The performance of the FeNi nanoparticles was significantly improved by annealing, with U-uptake increasing from 50 to 94%. Although the dissolution of Ni was completely inhibited by annealing, the Fe dissolution increased compared to that observed for the non-annealed FeNi nanoparticles, in contrast to behaviour exhibited by Fe-annealed nanoparticles. In all the systems, U was reduced to U(IV) and retained on the surfaces of the nanoparticulate solids for up to 48 h; the U-stability was not affected by annealing the Fe or the FeNi nanoparticles before use.






Similar content being viewed by others
References
US EPA Technical Factsheet on Nickel. http:www.epa.gov/safewater/dwh/t-ioc/nickel.html. (The Water Supply (Water Quality) Regulations 2000). viewed 02/10/08
Alowitz MJ, Scherer M (2002) Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ Sci Technol 36:299–306
Barnes RJ, Riba O, Gardner MN, Scott TB, Jackman SA, Thompson IP (2010) Optimization of nano-scale nickel/iron particles for the reduction of high concentration chlorinated aliphatic hydrocarbon solutions. Chemosphere 79:448–454
Bigg T, Judd SJ (2000) Zero-valent iron for water treatment. Environ Technol 21:661–670
Cao JS, Elliott D, Zhang WJJ (2005) Perchlorate reduction by nanoscale iron particles. Nanopart Res 7:499–506
Cheng R, Wang JL, Zhang WX (2007) Comparison of reductive dechlorination of p-chlorophenol using Fe-0 and nanosized Fe-0. J Hazard Mater 144:334–339
Choe S, Chang YY, Hwang KY, Khim J (2000) Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere 41:1307–1311
Crane RA, Dickinson M, Popescu IC, Scott TB (in review) The application of iron nanoparticles and vacuum annealed iron nanoparticles for the remediation of uranium contaminated waters from lisava, the Banat District, Romania
Dayta Systems Ltd, c/o Interface Analysis Centre, http://daytasystems.co.uk. Accessed 1 July 2010
Dickinson M, Scott TB (2010) The application of zero-valent iron nanoparticles for the remediation of a uranium-contaminated waste effluent. J Hazard Mater 178:171–179
Dickinson M, Scott TB, Crane RA, Barnes RJ, Hughes GM (2010) The effects of vacuum annealing on the structure and surface chemistry of iron nanoparticles. J Nanopart Res 12:2081–2092
Elliott DW, Zhang W (2001) Field assessment of nanoscale biometallic particles for groundwater treatment. Environ Sci Technol 35:4922–4926
Grittini C, Malcomson M, Fernando Q, Korte N (1995) Rapid dechlorination of polychlorinated-biphenyls on the surface of a Pd/Fe bimetallic system. Environ Sci Technol 29:2898–2900
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39:1291–1298
Li XQ, Zhang WX (2007) Sequestration of metal cations with zerovalent iron nanoparticles: a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phys Chem 111:6939–6946
Lien HL, Zhang WX (1999) Transformation of chlorinated methanes by nanoscale iron particles. J Environ Eng 125:1042–1047
Lien HL, Zhang WX (2001) Nanoscale iron particles for complete reduction of chlorinated ethenes. Colloids Surf A 191:97–105
Liu Y, Majetich SA, Tilton RD et al (2005) TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ Sci Technol 39:1338–1345
Miehr R, Tratnyek PG, Bandstra JZ et al (2004) Diversity of contaminant reduction reactions by zerovalent iron: role of the reductate. Environ Sci Technol 38:139–147
Mondal K, Jegadeesan G, Lalvani SB (2004) Removal of selenate by Fe and NiFe nanosized particles. Ind Eng Chem Res 43:4922–4934
Moura FCC, Oliveira GC, Araujo MH et al (2005) Formation of highly reactive species at the interface Fe degrees-iron oxides particles by mechanical alloying and thermal treatment: potential application in environmental remediation processes. Chem Lett 34:1172–1173
Nurmi JT, Tratnyek PG, Sarathy V et al (2005) Characterization and properties of metallic iron nanoparticles: spectroscopy, electrochemistry, and kinetics. Environ Sci Technol 39:1221–1230
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569
Ponder SM, Darab JG, Bucher J et al (2001) Surface chemistry and electrochemistry of supported zerovalent iron nanoparticles in the remediation of aqueous metal contaminants. Chem Mater 13:479–486
Riba O, Scott TB, Ragnarsdottir KV, Allen GC (2008) Reaction mechanism of uranyl in the presence of zero-valent iron nanoparticles. Geochim Cosmochim Ac 72:4047–4057
Schrick B, Blough JL, Jones AD, Mallouk TE (2002) Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-iron nanoparticles. Chem Mater 14:5140–5147
Scott TB (2005) Sorption of uranium onto iron bearing minerals. PhD thesis, University of Bristol
Scott TB, Allen GC, Heard PJ, Randall MG (2005) Reduction of U(VI) to U(IV) on the surface of magnetite. Geochim Cosmochim Ac 69:5639–5646
Scott TB, Dickinson M, Crane RA, Riba O, Hughes GM, Allen GC (2010) The effects of vacuum annealing on the structure and surface chemistry of iron nanoparticles. J Nanopart Res 12:1765–1775
Shimotori T, Nuxoll EE, Cussler EL, Arnold WA (2004) A polymer membrane containing Fe0 as a contaminant barrier. Environ Sci Technol 38:2264–2270
Tratnyek PG (1996) Putting corrosion to use: remediating contaminated groundwater with zero-valent metals. Chem Ind-London 1996(13):499–503
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
World Health Organisation:http://www.who.int/water_sanitation_health/dwq/gdwq0506_12.pdf. viewed Jan. 2008
Xu Y, Zhang W (2000) Subcolloidal Fe/Ag particles for reductive dehalogenation of chlorinated benzenes. Indus Eng Chem Res 39:2238
Zhang WX (2003) Nanoscale iron particles for environmental remediation: an overview. Nanopart Res 4:323–332
Zhang L, Manthiram A (1997) Chains composed of nanosize metal particles and identifying the factors driving their formation. Appl Phys Lett 70(18):2469–2471
Zhang WX, Wang CB, Lien HL (1998) Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal Today 40:387–395
Acknowledgments
The authors would like to thank Dr. Olga Riba of the Interface Analysis Centre, University of Bristol for manufacturing the nanoparticles and for valuable discussion. The authors also thank Dr. Chung Choi from the Department of Earth Sciences, University of Bristol for his help in performing the ICP-AES analysis. The authors thank Mr Philip Purdie and Dr. Sean Amos at AWE for facilitating the project. This research was funded by AWE plc under contract no. CDK0534/30002873.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dickinson, M., Scott, T.B. The effect of vacuum annealing on the remediation abilities of iron and iron−nickel nanoparticles. J Nanopart Res 13, 3699–3711 (2011). https://doi.org/10.1007/s11051-011-0291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0291-5