Abstract
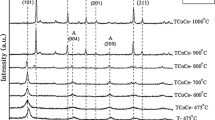
In this study, we report on the nanocrystalline powders of TiO2 and Fe-doped TiO2 (anatase and rutile phases) prepared by sol–gel method. The X-ray diffraction and Raman spectroscopy measurements indicated the presence of anatase or rutile phase in nanopowders. TEM micrographs showed 10 and 112 nm average particle sizes for anatase and rutile, respectively. Furthermore, their thermoluminescence properties were analyzed.








Similar content being viewed by others
References
Abazovic ND, Comor MI, Dramicanin MD, Jovanovic DJ, Ahrenkiel SP, Nedeljkovic JM (2006) Photoluminescence of anatase and rutile TiO2 particles. J Phys Chem B 110:25366–25370
Azorin-Vega JC, Azorin-Nieto J, García-Hipolito M, Rivera-Montalvo T (2007) Thermoluminescence properties of TiO2 nanopowder. Radiat Meas 42:613–616
Bahnemann DW, Hilgendorff M, Memming R (1997) Charge carrier dynamics at TiO2 particles: reactivity of free and trapped holes. J Phys Chem B 101:4265–4275
Bettinelli M, Speghini A, Falcomer D, Daldosso M, Dallacasa V, Roman L (2006) Photocatalytic, spectroscopic and transport properties of lanthanide-doped TiO2 nanocrystals. J Phys Condes Matter 18:S2149–S2160
Bruker AXS (2005) TOPAS V3: general profile and structure analysis software for powder diffraction data—User’s Manual. Bruker AXS, Karlsruhe
Buscema CL, Malibert C, Bach S (2002) Elaboration and characterization of thin films of TiO2 prepared by sol–gel process. Thin Solid Films 418:79–84
Cavalcante LS, Marques VS, Sczancoski JC, Escote MT, Joya MR, Varela JA, Santos MRMC, Pizani PS, Longo E (2008) Synthesis, structural refinement and optical behavior of CaTiO3 powders: a comparative study of processing in different furnaces. Chem Eng J 143:299–307
Cavalcante LS, Sczancoski JC, De Vicente FS (2009) Microstructure, dielectric properties and optical band gap control on the photoluminescence behavior of Ba(Zr0.25Ti0.75)O3 thin films. J Sol-Gel Sci Technol 46:35–46
Chaput F, Boilot JP, Beauger A (1990) Alkoxide–hydroxide route to synthesize BaTiO3-based powders. J Am Ceram Soc 73:942–948
Cullity BD (1978) Elements of X-ray diffraction. Addison-Wesley Publishing Co., Inc., Reading, pp 233, 296
Frindell KL, Bartl MH, Robinson MR, Bazan GC, Popitsch A, Stucky GD (2003) Visible and near IR luminescence via energy transfer in rare earth doped mesoporous titania thin films with nanocrystalline walls. J Solid State Chem 172:81–88
Furetta C, Weng PS (1998) Operational thermoluminescence dosimetry. World Scientific, Singapore
Howard CJ, Sabine TM, Dickson F (1991) Structural and thermal parameters for rutile and anatase. Acta Crystallogr: Struct Sci 47:462–468
Jin Y, Li G, Zhang Y, Zhang Y, Zhang L (2001) Photoluminescence of anatase TiO2 thin films achieved by the addition of ZnFe2O4. J Phys: Condens Matter 13:L913–L918(1)
Kim DH, Hong HS, Kim SJ, Song JS, Lee KS (2004) Photocatalytic behaviors and structural characterization of nanocrystalline Fe-doped TiO2 synthesized by mechanical alloying. J Alloys Compd 375:259–264
Kirsh Y (1992) Kinetic analysis of thermoluminescence. Phys Status Solidi (a) 129:15–48
Krishna KM, Rahman MM, Miki T, Soga T, Igarashi K, Tanemura S, Umeno M (1997) Optical properties of Pb doped TiO2 nanocrystalline thin films: a photoluminescence spectroscopic study. Appl Surf Sci 113–114:149–154
Lange S, Sildos I, Kiisk V, Aarik J (2004) Energy transfer in the photoexcitation of Sm3+-implanted TiO2 thin films. Mater Sci Eng B 112:87–90
Lj D, Arsov C, Kormann C, Plieth W (2005) Electrochemical synthesis and in situ Raman spectroscopy of thin films of titanium dioxide. J Raman Spectrosc 22:573–575
Longo VM, Cavalcante LS, Erlo R, Mastelaro VR, de Figueiredo AT, Sambrano JR, de Lazaro S, Freitas AZ, Gomes L, Vieira ND Jr et al (2008) Strong violet–blue light photoluminescence emission at room temperature in SrZrO3: joint experimental and theoretical study. Acta Mater 56:2191–2202
Lottici PP, Bersani D, Braghini M, Montenero A (1993) Raman scattering characterization of gel-derived titania glas. J Mater Sci 28:177–183
May CE, Patridge JA (1964) Thermoluminescence kinetics of alpha-irradiated alkali halides. J Chem Phys 40:1401–1409
Mizushima K, Tanaka M, Asai A, Iida S, Goodenough J (1979) Impurity levels of iron-group ions in TiO2(II). J Phys Chem Solids 40:1129–1140
Ohsake T, Izumi F, Fujiki Y (1978) Raman spectrum of anatase, TiO2. J Raman Spectrosc 7:321–324
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978–982
Prociow EL, Domaradzki J, Podhorodecki A, Borkowska A, Kaczmarek D, Misiewicz J (2007) Photoluminescence of Eu-doped TiO2 thin films prepared by low pressure hot target magnetron sputtering. Thin Solid Films 515:6344–6346
Rothenberger G, Moser J, Grätzel M (1985) Charge carrier trapping and recombination dynamics in small semiconductor particles. J Am Chem Soc 107:8054–8059
Stouwdam JW, van Veggel FCJM (2004) Sensitized emission in Ln3+-doped (Ln = Eu, Yb, Nd, and Er) semiconductor nanoparticles. Chem Phys Chem 5:743–746
Usami A (2000) Theoretical simulations of optical confinement in dye-sensitized nanocrystalline solar cells. Sol Energy Mater Sol Cells 64:73–83
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1997) Light-induced amphiphilic surfaces. Nature 388:431–432
Wang Z, Zu X, Xiang X, Yu H (2006) Photoluminescence from TiO2/PMMA nanocomposite prepared by γ radiation. J Nanoparticle Res 8:137–139
Zhang Z, Wang C, Zakaria R, Ying J (1998) Role of particle size in nanocrystalline TiO2-based photocatalysts. J Phys Chem B 102:10871–10878
Zhang WF, Zhang MS, Yin Z (2000) Microstructures and visible photoluminescence of TiO2 nanocrystals. Phys status solidi (a) 179:319–327
Acknowledgments
This study was supported by the “Nucleu”-project, PN09-450102, from the National plan for RDI, funded by the Romanian Ministry of Education and Research, and the National Authority for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cernea, M., Secu, M., Secu, C.E. et al. Structural and thermoluminescence properties of undoped and Fe-doped-TiO2 nanopowders processed by sol–gel method. J Nanopart Res 13, 77–85 (2011). https://doi.org/10.1007/s11051-010-0002-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0002-7