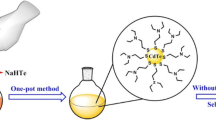
Abstract
A simple and general method has been proposed for preparing strong violet emitting CdS quantum dots, in which a ligand exchange strategy was applied to surface passivation and functionalization with good reproducibility. The resulting quantum dots showed a visible violet luminescence with emission peak centered near 423 nm and photoluminescence quantum yields reached over 30%. Additionally, different mercapto-compounds used as ligands can make different functionalized surfaces, favoring quantum dots dispersion in different media and their further applications. It was observed that the band edge emission has the main contribution to the bright violet luminescence.
Similar content being viewed by others
References
J. Aldana, N. Lavelle, Y. Wang, X. Peng 2005. Size-dependent dissociation pH of thiolate ligands from cadmium chalcogenide nanocrystals. J. Am. Chem. Soc. 127:2496
A.P. Alivisatos 1996. Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933
D. Battaglia, X. Peng 2002. Formation of high quality InP and InAs nanocrystals in a noncoordinating solvent. Nano Lett. 2:1027
W.C.W. Chan, S. Nie 1998. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016
A.R. Clapp, I.L. Medintz, J.M. Mauro, B.R. Fisher, M.G. Bawendi, H. Mattoussi 2004. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 126:301
A.R. Clapp, I.L. Medintz, H. Mattoussi 2006. Förster resonance energy transfer investigations using quantum-dot fluorophores. Chem. Phys. Chem. 7:47
R. Comparelli, F. Zezza, M. Striccoli, M.L. Curri, R. Tommasi, A. Agostiano 2003 Improved optical properties of CdS quantum dots by ligand exchange. Mater. Sci. Eng. C 23:1083
J.W. Eastman 1967. Quantitative spectrofluorimetry - the fluorescence quantum yield of quinine sulfate. Photochem. Photobiol. 6:55
F. Fleischhaker, R. Zentel 2005. Photonic crystals from core-shell colloids with incorporated highly fluorescent quantum dots. Chem. Mater. 17:1346
N. Gaponik, D. Talapin, H. Weller 2002. Thiol-capping of CdTe nanocrystals: an alternative to organometallic synthetic routes. J. Phys. Chem. B 106:7177
A. Henglein 1989. Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 89:1861
E. Jang, S. Jun, Y. Pu 2003. High quality CdSeS nanocrystals synthesized by facile single injection process and their electroluminescence. Chem. Commun. 24:2964
E. Jang, S. Jun, Y. Chung, L. Pu 2004. Surface treatment to enhance the quantum efficiency of semiconductor nanocrystals. J. Phys. Chem. B 108:4597
S. Jun, Jang E. 2005. Interfused semiconductor nanocrystals: brilliant blue photoluminescence and electroluminescence. Chem. Commun. 36:4616
S.Y. Lee, Harris M.T. 2006. Surface modification of magnetic nanoparticles capped by oleic acids:Characterization and colloidal stability in polar solvents. J. Colloid Interface Sci. 293:401
Y. Liu, M. Kim, Y. Wang, Y.A. Wang, X. Peng 2006. Highly luminescent, stable, and water-soluble CdSe/CdS core-shell dendron nanocrystals with carboxylate anchoring groups. Langmuir 22:6341
C.B. Murray, D.J. Norris, M.G. Bawendi 1993. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115:8706
D.J. Norris, M.G. Bawendi 1995. Measurement and assignment of the size-dependent optical spectrum in CdSe quantum dots. Phys. Rev. B 53:16338
N.M. Park, T.S. Kim, S.J. Park 2001. Band gap engineering of amorphous silicon quantum dots for light-emitting diodes. Appl. Phys. Lett. 78:2575
X. Peng, J. Wickham, A.P. Alivisatos 1998. Kinetics of II-VI and III-V colloidal semiconductor nanocrystal growth: “focusing” of size distributions. J. Am. Chem. Soc. 120:5343
M.M. Piepenbrock, S.M. Kelly, M. O’Neill 2006. A low-temperature synthesis for organically soluble HgTe nanocrystals exhibiting near-infrared photoluminescence and quantum confinement. J. Am. Chem. Soc. 128:7087
L. Qu, X. Peng 2002. Control of photoluminescence properties of CdSe nanocrystals in growth. J. Am. Chem. Soc. 124:2049
A. Shavel, N. Gaponik, A. Eychmuller 2004. Efficient UV-blue photoluminescing thiol-stabilized water-soluble alloyed ZnSe(S) nanocrystals. J. Phys. Chem. B 108:5905
J.S. Steckel, J.P. Zimmer, M.G. Bawendi 2004. Blue luminescence from (CdS)ZnS core-shell nanocrystals. Angew. Chem., Int. Ed. 43:2154
D.V. Talapin, A.L. Rogach, A. Kornowski, M. Haase, H. Weller 2001. Highly luminescent monodisperse CdSe and CdSe/ZnS nanocrystals synthesized in a hexadecylamine-trioctylphosphine oxide-trioctylphospine mixture. Nano Lett. 1:207
Q. Wang, D. Pan, X. Ji 2005. A new two-phase route to high-quality CdS nanocrystals. Chem. Eur. J. 11:3843
U. Wendy, J.J. Dittmer, A.P. Alivisatos 2002. Hybrid nanorod-polymer solar cells. Science 295:2425
N. Wu, L. Fu, M. Su, M. Aslam, K.C. Wong, V.P. Dravid 2004. Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett. 4:383
S.F. Wuister, I. Swart, F. van Driel, S.G. Hickey, C. de Mello Donega 2003. Highly luminescent water-soluble CdTe quantum dots. Nano Lett. 3:503
S. Xu, S. Kumar, T. Nann 2006. Rapid synthesis of high-quality InP nanocrystals. J. Am. Chem. Soc. 128:1054
W.W. Yu, L. Qu, W. Guo, X. Peng 2003. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 15:2854
W.W. Yu, J.C. Falkner, B.S. Shih, V.L. Colvin 2004. Preparation and characterization of monodisperse PbSe semiconductor nanocrystals in a noncoordinating solvent. Chem. Mater. 16:3318
W.W. Yu, X. Peng 2002. Formation of high-quality CdS and other II-VI semiconductor nanocrystals in noncoordinating solvents: tunable reactivity of monomers. Angew. Chem., Int. Ed. 41:2368
F. Zezza, R. Comparelli, M. Striccoli, M.L. Curri, R. Tommasi, A. Agostiano, M. Della Monica 2003 High quality CdS nanocrystals: surface effects. Synth. Met. 139:597
Z. Zhelev, R. Bakalova, H. Ohba, R. Jose, Y. Imai, Y. Baba 2006. Uncoated, broad fluorescent, and size-homogeneous CdSe quantum dots for bioanalyses. Anal. Chem. 78:321
B. Zorman, M.V. Ramakrishna, R.A. Friesner 1995. Quantum confinement effects in CdSe quantum Dots. J. Phys. Chem. 99:7649
Acknowledgments
The authors are grateful for the financial support from National Natural Science Foundation of China (Contract No.20575002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, TL., Xia, YS., Diao, XL. et al. Preparation and formation mechanism of strong violet luminescent CdS quantum dots by using a ligand exchange strategy. J Nanopart Res 10, 59–67 (2008). https://doi.org/10.1007/s11051-007-9221-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-007-9221-y