Abstract
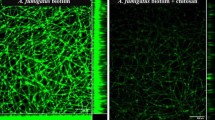
Invasive infections caused by filamentous fungi have increased considerably due to the alteration of the host's immune response. Aspergillus terreus is considered an emerging pathogen and has shown resistance to amphotericin B treatment, resulting in high mortality. The development of fungal biofilm is a virulence factor, and it has been described in some cases of invasive aspergillosis. In addition, although the general composition of fungal biofilms is known, findings related to biofilms of a lipid nature are rarely reported. In this study, we present the identification of a clinical strain of A. terreus by microbiological and molecular tools, also its in vitro biofilm development capacity: (i) Biofilm formation was quantified by Crystal Violet and reduction of tetrazolium salts assays, and simultaneously the stages of biofilm development were described by Scanning Electron Microscopy in High Resolution (SEM-HR). (ii) Characterization of the organizational structure of the biofilm was performed by SEM-HR. The hyphal networks developed on the surface, the abundant air channels created between the ECM (extracellular matrix) and the hyphae fused in anastomosis were described. Also, the presence of microhyphae is reported. (iii) The chemical composition of the ECM was analyzed by SEM-HR and CLSM (Confocal Laser Scanning Microscopy). Proteins, carbohydrates, nucleic acids and a relevant presence of lipid components were identified. Some structures of apparent waxy appearance were highlighted by SEM-HR and backscatter-electron diffraction, for which CLSM was previously performed. To our knowledge, this work is the first description of a lipid-type biofilm in filamentous fungi, specifically of the species A. terreus from a clinical isolate.




Similar content being viewed by others
References
Tekaia F, Latgé JP. Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol. 2005;8(4):385–92. https://doi.org/10.1016/j.mib.2005.06.017.
Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus-What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9(12):e1003743. https://doi.org/10.1371/journal.ppat.1003743.
Darling BA, Milder EA. Invasive aspergillosis. Pediatr Rev. 2018;39(9):476–8. https://doi.org/10.1542/pir.2017-0129.
Rudramurthy SM, Paul RA, Chakrabarti A, Mouton JW, Meis JF. Invasive Aspergillosis by Aspergillus flavus: epidemiology, diagnosis, antifungal resistance, and management. J Fungi (Basel). 2019;5(3):55. https://doi.org/10.3390/jof5030055.
Lass-Flörl C, Dietl AM, Kontoyiannis DP, Brock M. Aspergillus terreus Species Complex. Clin Microbiol Rev. 2021;34(4):e0031120. https://doi.org/10.1128/CMR.00311-20.
Lass-Flörl C. Treatment of infections due to Aspergillus terreus species complex. J Fungi (Basel). 2018;4(3):83. https://doi.org/10.3390/jof4030083.
Hachem R, Gomes MZ, El Helou G, El Zakhem A, Kassis C, Ramos E, Jiang Y, Chaftari AM, Raad II. Invasive aspergillosis caused by Aspergillus terreus: an emerging opportunistic infection with poor outcome independent of azole therapy. J Antimicrob Chemother. 2014;69(11):3148–55. https://doi.org/10.1093/jac/dku241.
Tritz DM, Woods GL. Fatal Disseminated Infection with Aspergillus terreus in Immunocompromised Hosts. Clin Infec Dis. 1993;16(1):118–22. https://doi.org/10.1093/clinids/16.1.118.
Mowat E, Williams C, Jones B, McChlery S, Ramage G. The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm? Med Mycol. 2009;47(Suppl 1):S120-126. https://doi.org/10.1080/13693780802238834.
Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother. 2011;55(5):2092–7. https://doi.org/10.1128/AAC.01189-10.
Ramírez-Granillo A, Bautista-Hernández LA, Bautista-De Lucío VM, Magaña-Guerrero FS, Domínguez-López A, Córdova-Alcántara IM, Pérez NO, Martínez-Rivera MLA, Rodríguez-Tovar AV. Microbial warfare on three fronts: mixed biofilm of Aspergillus fumigatus and Staphylococcus aureus on primary cultures of human limbo-corneal fibroblasts. Front Cell Infect Microbiol. 2021;11:646054. https://doi.org/10.3389/fcimb.2021.646054.
Raksha SG, Urhekar AD. Virulence factors detection in Aspergillus isolates from clinical and environmental samples. J Clin Diagn Res. 2017;11(7):13–8.
Branda SS, Vik A, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–6. https://doi.org/10.1016/j.tim.2004.11.006.
Guarro J, Orzechowski M, Severo X, Severo LC. Differences and similarities Amongst pathogenic Aspergillus species. In: Pasqualotto AC, editor. Aspergillosis: from diagnosis to prevention. Porto Alegre, Brazil: Springer; 2010. p. 7–32.
Larone DH, Walsh TJ, Hayden RT. Larone’s medically important fungi: a guide to identification. 6th ed., Washington, DC: ASM Press, 2018. pp. 44, 307. https://doi.org/10.1128/9781555819880.
Rodríguez-Tovar AV, Ruiz-Medrano R, Herrera-Martínez A, Barrera-Figueroa BE, Hidalgo-Lara ME, Reyes-Márquez BE, Cabrera-Ponce JL, Valdés M, Xoconostle-Cázares B. Stable genetic transformation of the ectomycorrhizal fungus Pisolithus tinctorius. J Microbiol Methods. 2005;63(1):45–54. https://doi.org/10.1016/j.mimet.2005.02.016.
Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–8. https://doi.org/10.1111/j.1365-294x.1993.tb00005.x.
Peterson SW. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100(2):205–26. https://doi.org/10.3852/mycologia.100.2.205.
Tsang CC, Tang JYM, Lau SKP, Woo PCY. Taxonomy and evolution of Aspergillus, Penicillium and Talaromyces in the omics era–Past, present and future. Comput Struct Biotechnol J. 2018;16:197–210. https://doi.org/10.1016/j.csbj.2018.05.003.
Mowat E, Rajendran R, Williams C, McCulloch E, Jones B, Lang S, Ramage G. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbial Lett. 2010;313(2):96–102. https://doi.org/10.1111/j.1574-6968.2010.02130.x.
Ramírez-Granillo A, Canales MG, Espíndola ME, Martínez Rivera MA, de Lucio VM, Tovar AV. Antibiosis interaction of Staphylococcus aureus on Aspergillus fumigatus assessed in vitro by mixed biofilm formation. BMC Microbiol. 2015;15:33. https://doi.org/10.1186/s12866-015-0363-2.
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996–1006. https://doi.org/10.1128/jcm.22.6.996-1006.1985.
Walencka E, Sadowska B, Rozalska S, Hryniewicz W, Rózalska B. Lysostaphin as a potential therapeutic agent for staphylococcal biofilm eradication. Pol J Microbiol. 2005;54(3):191–200.
Ramírez-Granillo A. Analysis of the antagonistic interaction between Aspergillus fumigatus and Staphylococcus aureus during biofilm formation. PhD thesis, Escuela Nacional de Ciencias Biológicas-Instituto Politécnico Nacional; 2017.
Camarillo-Márquez O, Córdova-Alcántara IM, Hernández-Rodríguez C, García-Pérez BE, Martínez-Rivera M, Rodríguez-Tovar AV. Antagonistic interaction of Staphylococcus aureus toward Candida glabrata during in vitro biofilm formation is caused by an Apoptotic mechanism. Front Microbiol. 2018;9:2031. https://doi.org/10.3389/fmicb.2018.02031.
Bozzola JJ, Russell LD. Specimen preparation for scanning electron microscopy. In: electron microscopy: principles and techniques for biologists. 2nd edn. New York, USA: Jones and Bartlett Press. 1999:48–71.
Vazquez-Nin G, Echeverría. Introduction to the electronic microscopy applied to the biological sciences, [Introducción a la microscopia electrónica aplicada a las ciencias biológicas]. CDMX, México: Fondo de Cultura Económica, 2000.
Allkja J, Bjarnsholt T, Coenye T, Cos P, Fallarero A, Harrison JJ, Lopes SP, Oliver A, Pereira MO, Ramage G, Shirtliff ME, Stoodley P, Webb JS, Zaat SAJ, Goeres DM, Azevedo NF. Minimum information guideline for spectrophotometric and fluorometric methods to assess biofilm formation in microplates. Biofilm. 2019;2:100010. https://doi.org/10.1016/j.bioflm.2019.100010.
Fakhim H, Badali H, Dannaoui E, Nasirian M, Jahangiri F, Raei M, Vaseghi N, Ahmadikia K, Vaezi A. Trends in the prevalence of Amphotericin B-Resistance (AmBR) among Clinical Isolates of Aspergillus Species. J Mycol Med. 2022;32(4):101310. https://doi.org/10.1016/j.mycmed.2022.101310.
Vaezi A, Fakhim H, Arastehfar A, et al. In vitro antifungal activity of amphotericin B and 11 comparators against Aspergillus terreus species complex. Mycoses. 2018;61(2):134–42. https://doi.org/10.1111/myc.12716.
Zoran T, Sartori B, Sappl L, Aigner M, Sánchez-Reus F, et al. Azole-Resistance in Aspergillus terreus and Related Species: an emerging problem or a rare phenomenon? Front Microbiol. 2018;9:516. https://doi.org/10.3389/fmicb.2018.00516.
Kathuria S, Sharma C, Singh PK, Agarwal P, Agarwal K, Hagen F, Meis JF, Chowdhary A. Molecular epidemiology and in-vitro antifungal susceptibility of Aspergillus terreus species complex isolates in Delhi, India: evidence of genetic diversity by amplified fragment length polymorphism and microsatellite typing. PLoS ONE. 2015;10(3):e0118997. https://doi.org/10.1371/journal.pone.0118997.
Damek DM, Lillehei KO, Kleinschmidt-DeMasters BK. Aspergillus terreus brain abscess mimicking tumor progression in a patient with treated glioblastoma multiforme. Clin Neuropathol. 2008;27(6):400–7. https://doi.org/10.5414/npp27400.
Elsawy A, Faidah H, Ahmed A, Mostafa A, Mohamed F. Aspergillus terreus Meningitis in Immunocompetent Patient: A Case Report. Front Microbiol. 2015;6:1353. https://doi.org/10.3389/fmicb.2015.01353.
Srikumar T, Pabbathi S, Fernandez J, Nanjappa S. Aspergillus Terreus brain abscess complicated by tension Pneumocephalus in a patient with Angiosarcoma. Am J Case Rep. 2017;18:33–7.
Marzolf G, Sabou M, Lannes B, Cotton F, Meyronet D, Galanaud D, Cottier JP, Grand S, Desal H, Kreutz J, Schenck M, Meyer N, Schneider F, Dietemann JL, Koob M, Herbrecht R, Kremer S. Magnetic resonance imaging of cerebral aspergillosis: imaging and pathological correlations. PLoS One. 2016;11(4):0152475. https://doi.org/10.1371/journal.pone.0152475.
De Anda-Gómez MA, Díaz-Ponce H, Pacheco-Rosas DO, Reséndiz-Sánchez J, Sandoval-Mex AM. Diagnosis Of Invasive Aspergillosis In Children Under 18 Years Of Age, [Diagnóstico de Aspergilosis Invasora en menores de 18 años de edad]. CDMX, Mexico: Instituto Mexicano del Seguro Social. 2013.
Cruz-Contreras DG. Invasive aspergillosis in the patient receiving an allogeneic hematopoietic progenitor cell transplant: epidemiology, diagnosis, prophylaxis and treatment, [Aspergilosis invasiva en el paciente que recibe trasplante alogénico de células progenitoras hematopoyéticas: epidemiología, diagnóstico, profilaxis y tratamiento]. Rev Hematol Mex. 2016;17(4):262–7.
Vergara GE, Roura N, Del Castillo M, Mora A, Alcorta SC, Mormandi R, Cervio A, Salvat J. Cervical aspergillosis with dissemination to the central nervous system: Case reports and review of the literature. [Aspergilosis cervical con diseminación al sistema nervioso central. Presentación de un caso y revisión de bibliografía]. Surg Neurol Int. 2015;6(Suppl 20):S524-S529; https://doi.org/10.4103/2152-7806.167203.
Sangrador-Deitos MV, Olvera J, Espinal HA, Hernández GC, Morales VA, Soto Hernandez JL. Fungal mycotic aneurysm in a patient with Aspergillus terreus chronic meningoencephalitis. Surg Neurol Int. 2020;11:139. https://doi.org/10.25259/SNI_506_2019.
Raksha GS, Urhekar AD. Virulence factors detection in Aspergillus isolates from clinical and environmental samples. J Cli Diagn Res. 2017;11(7):DC13–8.
Lass-Flörl C, Griff K, Mayr A, Petzer A, Gastl G, Bonatti H, Freund M, Kropshofer G, Dierich MP, Nachbaur D. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br J Haematol. 2005;131(2):201–7. https://doi.org/10.1111/j.1365-2141.2005.05763.x.
Thakur R, Shankar J. Proteome profile of Aspergillus terreus conidia at germinating stage: identification of probable virulent factors and enzymes from mycotoxin pathways. Mycopathologia. 2017;182:771–84. https://doi.org/10.1007/s11046-017-0161-5.
Bengyella L, Yekwa EL, Subhani MN, Tambo E, Nawaz K, Hetsa BA, Iftikhar S, Waikhom SD, Roy P. Invasive Aspergillus terreus morphological transitions and immunoadaptations mediating antifungal resistance. Infect Drug Resist. 2017;10:425–36. https://doi.org/10.2147/IDR.S147331.
Won EJ, Choi MJ, Shin JH, Park YJ, Byun SA, Jung JS, Kim SH, Shin MG, Suh SP. Diversity of clinical isolates of Aspergillus terreus in antifungal susceptibilities, genotypes and virulence in Galleria mellonella model: comparison between respiratory and ear isolates. PLoS ONE. 2017;12(10):e0186086. https://doi.org/10.1371/journal.pone.0186086.
Shin WS, Lee D, Kim S, Jeong YS, Chun GT. Development of miniaturized culture systems for large screening of mycelial fungal cells of Aspergillus terreus producing itaconic acid. J Microbiol Biotechnol. 2017;27(1):101–11. https://doi.org/10.4014/jmb.1610.10037.
Wuren T, Toyotome T, Yamaguchi M, Takahashi-Nakaguchi A, Muraosa Y, Yahiro M, Wang DN, Watanabe A, Taguchi H, Kamei K. Effect of serum components on biofilm formation by Aspergillus fumigatus and other Aspergillus species. Jpn J Infect Dis. 2014;67(3):172–9. https://doi.org/10.7883/yoken.67.172.
Gutiérrez-Correa M, Ludeña Y, Ramage G, Villena GK. Recent advances on filamentous fungal biofilms for industrial uses. Appl Biochem Biotechnol. 2012;167(5):1235–53. https://doi.org/10.1007/s12010-012-9555-5.
Kaur S, Singh S. Biofilm formation by Aspergillus fumigatus. Med Mycol. 2014;52:2–9. https://doi.org/10.3109/13693786.2013.819592.
González-Ramírez AI, Ramírez-Granillo A, Medina-Canales MG, Rodríguez-Tovar AV, Martínez-Rivera MA. Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol. 2016;16(1):243. https://doi.org/10.1186/s12866-016-0859-4.
Kulkarni G. Introduction to fermentation technology. in: biotechnology and its applications in pharmacy. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2002:7.
Srinivasan N, Thangavelu K, Uthandi S. Lovastatin production by an oleaginous fungus, Aspergillus terreus KPR12 using sago processing wastewater (SWW). Microb Cell Fact. 2022;21(1):22. https://doi.org/10.1186/s12934-022-01751-2.
Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, Paris S, Mallet A, Prévost MC, Latgé JP. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9(6):1588–600. https://doi.org/10.1111/j.1462-5822.2007.00895.x.
Harding MW, Marques LL, Howard RJ, Olson ME. Can filamentous fungi form biofilms? Trends microbiol. 2009;17(11):475–80. https://doi.org/10.1016/j.tim.2009.08.007.
Ramage G, Rajendran R, Gutiérrez-Correa M, Jones B, Williams C. Aspergillus biofilms: clinical and industrial significance. FEMS Microbial Lett. 2011;324(2):89–97. https://doi.org/10.1111/j.1574-6968.2011.02381.x.
Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog. 2012;8(4):e1002585. https://doi.org/10.1371/journal.ppat.1002585.
Blanchette, RA, Biggs AR, editors. Defense Mechanisms of Woody Plants Against Fungi. Springer Series in Wood Science. Berlin: Springer, 1992; https://doi.org/10.1007/978-3-662-01642-8.
Gašparíková O, Mistrík I, Čiamporová M. Waisel, Y, Eshel A., Kafkafi U, editors. In: Plant roots. The Hidden Half, Edition: 3rd ed. Chapter: Sieber T.N. Fungal Root Endophytes. Publisher: Marcel Dekker, New York: pp. 887–917; https://doi.org/10.1093/aob/mcf252.
Ouellette GB, Baayen RP, Rioux D, Simard M. Peculiar ultrastructural characteristics of fungal cells and of other elements apposed to and in vessel walls in plants of a susceptible carnation cultivar, infected with Fusarium oxysporum f.sp. dianthi race 2. Phytoprotection. 2004; 85(3):121–138; https://doi.org/10.7202/010905ar.
Padhi S, Uppin SG, Uppin MS, Umabala P, Challa S, Laxmi V, Prasad VB. Mycetoma in South India: retrospective analysis of 13 cases and description of two cases caused by unusual pathogens: Neoscytalidium dimidiatum and Aspergillus flavus. Int J Dermatol. 2010;49(11):1289–96. https://doi.org/10.1111/j.1365-4632.2010.04610.x.
Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–33. https://doi.org/10.1038/nrmicro2415.
Shopova I, Bruns S, Thywissen A, Kniemeyer O, Brakhage AA, Hillmann F. Extrinsic extracellular DNA leads to biofilm formation and colocalizes with matrix polysaccharides in the human pathogenic fungus Aspergillus fumigatus. Front Microbiol. 2013;4:141. https://doi.org/10.3389/fmicb.2013.00141.
Mesa-Grajales DH. Principles and applications of the Electron Back-Scattering Diffraction Tecnical (EBSD). [Principios y aplicaciones de la técnica de difracción de electrones retro-proyectados]. Informador Técnico. 2010;74:64–74; https://doi.org/10.23850/22565035.9.
Chen H, Yao Y, Warner JA, Qu J, Yun F, Ye Z, Ringer SP, Zheng R. Grain size quantification by optical microscopy, electron backscatter diffraction, and magnetic force microscopy. Micron. 2017;101:41–7. https://doi.org/10.1016/j.micron.2017.06.001.
Casas-López JL. Production of lovastatin from Aspergillus terreus in a fluidized bed reactor. PhD thesis, Universidad de Almería, 2004.
Hu Z, He B, Ma L, Sun Y, Niu Y, Zeng B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces cerevisiae. Indian J Microbiol. 2017;57(3):270–7. https://doi.org/10.1007/s12088-017-0657-1.
Acknowledgements
Our sincere thanks to Secretaria de Investigación y Posgrado of ENCB, and the Centro de Estudios Científicos y Tecnológicos N° 6 "Miguel Othón de Mendizabal", IPN. GRL wishes to thank BEIFI/Instituto Politécnico Nacional fellowship program. MGMC, BEGP, NOP, AVRT and ARG are SNI members. AVRT and BEGP are EDI, COFAA/ Instituto Politécnico Nacional and SNI/CONACYT Mexico fellows. ARG is COFAA/ Instituto Politécnico Nacional and SNI/CONACYT Mexico fellow. A special thanks to Dr. Hugo Martínez Gutiérrez for SEM photographs carried out in Laboratory of Nanosciences, Micro and Nanotechnology Center-IPN. Authors wish to thank Hospital Infantil de México “Dr. Federico Gómez”, Mexico City for providing the clinical isolate for this project. The figure 3 was created with BioRender.com.
Funding
This work was supported by Instituto Politécnico Nacional, Mexico City [SIP20210778, SIP20210200, SIP20220564, SIP20221965]. Author GRL has receives research support from BEIFI/Instituto Politécnico Nacional. Authors AVRT and ARG have received research support from EDI, COFAA/Instituto Politécnico Nacional and SNI/CONACYT México.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by G Rayón-López, N Carapia-Minero, MG Medina-Canales, BE García-Pérez, J Reséndiz-Sánchez, NO. Pérez, AV Rodríguez-Tovar and A Ramírez-Granillo. The first draft of the manuscript was written by G Rayón-López and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Handling Editor: Hamid Badali.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11046_2022_692_MOESM1_ESM.pdf
Online Resource 1. Growth kinetics of A. terreus on solid medium. Colonies were measured with an inoculum of 1x108 conidia/mL for the construction of the graphic after 7 days of incubation. The fungus was grown on PDA and SDA, at 28°C (a) and 37°C (b). A. terreus was grown more efficiently at 28°C for 7 days with no significant difference with the use of different culture media. In contrast, inferior growth was observed at 37°C using SDA medium. Student Neumals Kewls statistical method was used for analysis (n=12; (*): p<0.05). (PDF 43 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rayón-López, G., Carapia-Minero, N., Medina-Canales, M.G. et al. Lipid-Like Biofilm from a Clinical Brain Isolate of Aspergillus terreus: Quantification, Structural Characterization and Stages of the Formation Cycle. Mycopathologia 188, 35–49 (2023). https://doi.org/10.1007/s11046-022-00692-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-022-00692-z