Abstract
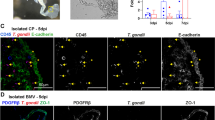
Paracoccidioidomycosis (PCM), a systemic mycosis caused by the fungus Paracoccidioides spp. is the most prevalent fungal infection among immunocompetent patients in Latin America. The estimated frequency of central nervous system (CNS) involvement among the human immunodeficiency virus (HIV)/PCM-positive population is 2.5%. We aimed to address the impact of neuroparacoccidioidomycosis (NPCM) and HIV/NPCM co-infection on the tight junctions (TJ) and adherens junction (AJ) proteins of the CNS. Four CNS formalin-fixed paraffin-embedded (FFPE) tissue specimens were studied: NPCM, NPCM/HIV co-infection, HIV-positive without opportunistic CNS infection, and normal brain autopsy (negative control). Immunohistochemistry was used to analyze the endothelial cells and astrocytes expressions of TJ markers: claudins (CLDN)-1, -3, -5 and occludin; AJ markers: β-catenin and E-cadherin; and pericyte marker: alpha-smooth muscle actin. FFPE CNS tissue specimens were analyzed using the immunoperoxidase assay. CLDN-5 expression in the capillaries of the HIV/NPCM coinfected tissues (mixed clinical form of PCM) was lower than that in the capillaries of the HIV or NPCM monoinfected (chronic clinical form of PCM) tissues. A marked decrease in CLDN-5 expression and a compensatory increase in CLDN-1 expression in the NPCM/HIV co-infection tissue samples was observed. The authors suggest that Paracoccidioides spp. crosses the blood–brain barrier through paracellular pathway, owing to the alteration in the CLDN expression, or inside the macrophages (Trojan horse).



Similar content being viewed by others
Availability of data and material
Data and material are available after approval of the institutional review board and request to the corresponding author.
References
Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F, Kono ASG, Paniago AMM, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop. 2017;50:715–40.
Goldani LZ, Sugar AM. Paracoccidioidomycosis and AIDS: an overview. Clin Infect Dis. 1995;21:1275–81.
Benard G, Duarte AJ. Paracoccidioidomycosis: a model for evaluation of the effects of humanimmunodeficiency virus infection on the natural history of endemic tropical diseases. Clin Infect Dis. 2000;31:1032–9.
Mendes RP, Cavalcante RS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, et al. Paracoccidioidomycosis: current perspectives from Brazil. Open Microbiol J. 2017;11:224–82.
de Almeida SM, Roza TH, Salvador GLO, França JCB, Vidal LRR, Nogueira MB, Oliva LV, Torres LFB, de Noronha L. Neurological and multiple organ involvement due to Paracoccidioides brasiliensis and HIV co-infection diagnosed at autopsy. J Neurovirol. 2017;23:913–8.
Nag S, et al. Review: molecular pathogenesis of blood–brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37:3–23.
Atluri VS, Hidalgo M, Samikkannu T, Kurapati KRV, Jayant RD, Sagar V, et al. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci. 2015;9:212.
Liu WY, Wang ZB, Zhang LC, Wei X, Li L. Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther. 2012;18:609–15.
Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–27.
Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of TJ in the blood-brain barrier. Trends Neurosci. 2001;24:719–25.
Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–19.
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: An overview– structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13.
Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9.
Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899.
Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209:493–506.
Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers. 2016;4(1):e1154641.
Vorbrodt AW, Dobrogowska DH. Molecular anatomy of interendothelial junctions in human blood–brain barrier microvessels. Folia Histochem Cytobiol. 2004;42:67–75.
Xu R, Feng X, Xie X, Zhang J, Wu D, Xu L. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occluding via matrix metallo proteinase-9. Brain Res. 2012;1436:13–9.
de Almeida SM, Queiroz-Telles F, Doi EM, Ono M, Werneck LC. Anti-gp43 antibodies in the cerebrospinal fluid of patients with central nervous system involvement by paracoccidioidomycosis. Am J Clin Pathol. 2002;118:864–8.
Teive HAG, Zanatta Al, Germiniani FMB, de Almeida SM, Werneck LC. Holmes' tremor and neuroparacoccidioidomycosis: A case report. Movement Disorders 2002; 17:1392–4.
Rosa Júnior M, Amorim AC, Baldon IV, Martins LA, Pereira RM, Campos RP, Gonçalves SS, Velloso TRG, Peçanha P, Falqueto A. Paracoccidioidomycosis of the Central Nervous System: CT and MR Imaging Findings. Am J Neuroradiol. 2019;40:1681–8.
Andersson LM, Hagberg L, Fuchs D, Svennerholm B, Gisslen M. Increased blood–brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals, correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J NeuroVirol. 2001;7:542–7.
Calcagno A, Alberione MC, Romito A, Imperiale D, Ghisetti V, Audagnotto S, Lipani F, Raviolo S, Di Perri G, Bonora S. Prevalence and predictors of blood–brain barrier damage in the HAART era. J Neurovirol. 2014;20:521–5.
de Almeida SM, Rotta I, Ribeiro CE, Smith D, Wang R, Judicello J, Potter M, Vaida F, Letendre S, Ellis RJ, HNRC Group. Blood-CSF barrier and compartmentalization of CNS cellular immune response in HIV infection. J Neuroimmunol. 2016;301:41–8.
Calcagno A, Motta I, Ghisetti V, Lo Re S, Allice T, Marinaro L, Milia MG, Tettoni MC, Trentini L, Orofino G, Salassa B, Di Perri G, Bonora S. HIV-1 very low level viremia is associated with virological failure in highly active antiretroviral treatment-treated patients. AIDS Res Hum Retrov. 2015;31:999–1008.
Marshall DW, Brey RL, Butzin CA, Lucey DR, Abbadessa SM, Boswell RN. CSF changes in a longitudinal study of 124 neurologically normal HIV-1-infected U.S. Air Force personnel. J Acquir Immune Defic Syndr. 1991;4:777–81.
McArthur JC, Nance-Sproson TE, Griffin DE, Hoover D, Selnes OA, Miller EN, Margolick JB, Cohen BA, Farzadegan H, Saah A. The diagnostic utility of elevation in cerebrospinal fluid beta 2-microglobulin in HIV-1 dementia. Multicenter AIDS Cohort Study Neurol. 1992;42:1707–12.
Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel FD, Pfister HW. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–7.
de Almeida SM, Rotta I, Ribeiro CE, Oliveira MF, Chaillon A, de Pereira AP, et al. Dynamic of CSF and serum biomarkers in HIV-1 subtype C encephalitis with CNS genetic compartmentalization: case study. J Neurovirol. 2017;23:460–73.
de Almeida SM, Oliveira MF, Chaillon A, Rotta I, Ribeiro CE, de Pereira AP, et al. Transient and asymptomatic meningitis in human immunodeficiency virus-1 subtype C: a case study of genetic compartmentalization and biomarker dynamics. J Neurovirol. 2018;24:786–96.
Giovannoni G, Miller RF, Heales SJ, Land JM, Harrison MJ, Thompson EJ. Elevated cerebrospinal fluid and serum nitrate and nitrite levels in patients with central nervous system complications of HIV-1 infection: a correlation with blood-brain-barrier dysfunction. J Neurol Sci. 1998;156:53–8.
Strazza M, et al. Breaking down the barrier: The effects of HIV-1 on the blood–brain barrier. Brain Res. 2011;1399:96–115.
Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505.
Acheampong E, Mukhtar M, Parveen Z, Ngoubilly N, Ahmad N, Patel C, et al. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology. 2002;304:222–34.
Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186:S193–8.
Brasil, Ministério da Saúde. Programa Nacional de DST/AIDS. 2018. http://www.aids.gov.br/assistencia/manualdst/item12.htm.
Dore-Duffy P, Mehedi A, Wang X, et al. Immortalized CNS pericytes are quiescent smooth muscle actin-negative and pluripo-tent. Microvasc Res. 2011;82:18–27.
de Souza Costa VH, Baurakiades E, Viola Azevedo ML, Traiano G, Kowal Rosales J, Kunze Larsen KS, et al. Immunohistochemistry analysis of pulmonary infiltrates in necropsy samples of 600 children with non-pandemic lethal respiratory infections (RSV; ADV; PIV1; PIV2; PIV3; FLU A; FLU B). J Clin Virol. 2014;61:211–5.
Chong DC, Raboni SM, Abujamra KB, Marani DM, Nogueira MB, Tsuchiya LRV, et al. Respiratory viruses in pediatric necropsies: an immunohistochemical study. Pediatr Dev Pathol. 2009;12:211–6.
Okamoto C, Bahr J, Silva L, Noronha L. Análises histopatológica e morfométrica no diagnóstico da “nova” displasia broncopulmonar e comparação clinicopatológica com a forma clássica da doença. J Bras Patol Med Lab. 2009;45:155–60.
Harvey J, Clark G, Osborne C, Allred D. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–781.
Sladojevic N, Stamatovic SM, Johnson AM, Choi J, Hu A, Dithmer S, Blasig IE, Keep RF, Andjelkovic AV. Claudin-1-dependent destabilization of the blood-brain barrier in chronic stroke. J Neurosci. 2019;39:743–57.
Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, Rausch V, Blasig R, Richter M, Sporbert A, Wolburg H, Blasig IE, Haseloff RF. Tight junction proteins at the blood–brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019;76:1987–2002.
Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–31.
Shin JS, Hyun SY, Kim DH, Lee S, Jung JW, Choi JW, Ko KH, Kim JM, Ryu JH. Chronic hypoperfusion increases claudin-3 immunoreactivity in rat brain. Neurosci Lett. 2008;445:144–8.
Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier TJ ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–14.
Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, et al. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1encephalitis. Am J Pathol. 1999;155:1915–27.
Mahajan SD, et al. Tight junction regulation by morphine and HIV-1 tat modulates blood–brain barrier permeability. J Clin Immunol. 2008;28:528–41.
Andras IE, Pu H, Deli MA, Nath A, Henning B, Toborek M. HIV-1 Tat protein alters tight unction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–65.
Xu R, et al. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res. 2011;1436:13–9.
Sardo L, Vakil PR, Elbezanti W, El-Sayed A, Klase Z. The inhibition of microRNAs by HIV-1 Tat suppresses beta catenin activity in astrocytes. Retrovirology. 2016;13:25.
Louboutin JP, Strayer DS. Blood-Brain Barrier Abnormalities Caused by HIV-1 gp120: mechanistic and Therapeutic Implications. Scientific World Journal. 2012, Article ID 482575, 1–15
Louboutin JP, Agrawal L, Reyes BA, VanBockstaele EJ, Strayer DS. HIV-1gp120-induced injury to the blood-brain barrier:role of metallo proteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol. 2010;69:801–16.
Kanmogne GD, et al. HIV-1 gp120 compromises blood–brain barrier integrity and enhances monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–34.
Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency vírus type 1 vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–26.
Sporer B, Koedel U, Paul R, Kohleisen B, Erfle V, Fontana A, Pfisteret HW, al. Human immunodeficiency virus type-1 Nef protein induces blood-brain barrier disruption in the rat: role of matrix metalloproteinase-9. J Neuroimmunol. 2000; 102:125–30
Weiser K, Barton M, Gershoony D, DasGupta R, Cardozo T. HIV’s Nef interacts with β-catenin of the Wnt signaling pathway in HEK293 cells. PLoS ONE. 2013;8:e77865. https://doi.org/10.1371/journal.pone.0077865.
de Almeida SM, Roza TH, Salvador GLO, Izycki LF, Locatelli G, Santos ID, Aragão A, Torres LFB, de Noronha L. Autopsy and biopsy study of paracoccidioidomycosis and neuroparacoccidioidomycosis with and without HIV co-infection. Mycoses. 2018;61:237–44.
Colombo AL, Junior GT, Lotfi CJ, Lima FO, Levi DS, Accerturi CA. Paracoccidioidomicose disseminada em pacientes com AIDS (achado de necropsia) [abstract]. In: Program and abstracts of the 26th Congresso da Sociedade Brasileira de Medicina Tropical (São Paulo). São Paulo: Sociedade Brasileira de Medicina Tropical, 1989.
Guimarães JCA, Bortoliero AI, Bonametti AM, et al. Infecção oportunista do sistema nervoso central por Paracoccidioides brasiliensis: relato do caso [abstract no 114]. Rev Soc Bras Med Trop. 1991;24:30–1.
Finamor LP, Muccioli C, Martins MC, Rizzo LV, Belfort R. Ocular and central nervous system paracoccidioidomycosis in a pregnant woman with acquired immunodeficiency syndrome. Am J Ophthalmol. 2002;134:456–9.
Silva-Vergara ML, Rocha IH, Vasconcelos RR, Maltos AL, de Freitas NF, de Almeida STL, Mora DJ. Central nervous system paracoccidioidomycosis in an AIDS patient: case report. Mycopathologia. 2014;177:137–41.
Camacho E, Niño-Vega GA. Paracoccidioides Spp.: virulence factors and immune-evasion strategies. Mediat Inflamm. 2017;2017:531–691. https://doi.org/10.1155/2017/5313691.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SMdA: conceived and designed the study, performed research, analyzed data, contributed new methods or models, wrote the paper. AK: performed research. MM: performed research. SN: performed research. CdP: performed research. MM: conceived the study. LdN: conceived and designed the study, performed research, analyzed data, contributed new methods or models, wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling Editor: Anamelia Lorenzetti Bocca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida, S.M., Kulik, A., Malaquias, M.A.S. et al. The Impact of Paracoccidioides spp Infection on Central Nervous System Cell Junctional Complexes. Mycopathologia 187, 567–577 (2022). https://doi.org/10.1007/s11046-022-00653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-022-00653-6