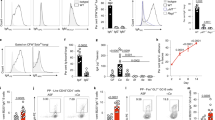
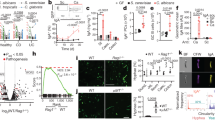
Abstract
The details of how gut-associated lymphoid tissues such as Peyer’s patches (PPs) in the small intestine play a role in immune surveillance, microbial differentiation and the mucosal barrier protection in response to fungal organisms such as Candida albicans are still unclear. We particularly focus on PPs as they are the immune sensors and inductive sites of the gut that influence inflammation and tolerance. We have previously demonstrated that CD11c+ phagocytes that include dendritic cells and macrophages are located in the sub-epithelial dome within PPs sample C. albicans. To gain insight on how specific cells within PPs sense and respond to the sampling of fungi, we gavaged naïve mice with C. albicans strains ATCC 18804 and SC5314 as well as Saccharomyces cerevisiae. We measured the differential gene expression of sorted CD45+ B220+ B-cells, CD3+ T-cells and CD11c+ DCs within the first 24 h post-gavage using nanostring nCounter® technology. The results reveal that at 24 h, PP phagocytes were the cell type that displayed differential gene expression. These phagocytes were able to sample C. albicans and discriminate between strains. In particular, strain ATCC 18804 upregulated fungal-specific pro-inflammatory genes pertaining to innate and adaptive immune responses. Interestingly, PP CD11c+ phagocytes also differentially expressed genes in response to C. albicans that were important in the protection of the mucosal barrier. These results highlight that the mucosal barrier not only responds to C. albicans, but also aids in the protection of the host.






Similar content being viewed by others
References
Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14(4):386–91. https://doi.org/10.1016/j.mib.2011.07.015.
Tong Y, Tang J. Candida albicans infection and intestinal immunity. Microbiol Res. 2017;198:27–35. https://doi.org/10.1016/j.micres.2017.02.002.
Bohringer M, Pohlers S, Schulze S, Albrecht-Eckardt D, Piegsa J, Weber M, et al. Candida albicans infection leads to barrier breakdown and a MAPK/NF-kappaB mediated stress response in the intestinal epithelial cell line C2BBe1. Cell Microbiol. 2016;18(7):889–904. https://doi.org/10.1111/cmi.12566.
Liguori G, Lamas B, Richard ML, Brandi G, da Costa G, Hoffmann TW, et al. Fungal dysbiosis in Mucosa-associated microbiota of crohn’s disease patients. J Crohns Colitis. 2016;10(3):296–305. https://doi.org/10.1093/ecco-jcc/jjv209.
Frykman PK, Nordenskjold A, Kawaguchi A, Hui TT, Granstrom AL, Cheng Z, et al. Characterization of bacterial and fungal microbiome in children with Hirschsprung disease with and without a history of enterocolitis: a multicenter study. PLoS ONE. 2015;10(4):e0124172. https://doi.org/10.1371/journal.pone.0124172.
Jawhara S, Thuru X, Standaert-Vitse A, Jouault T, Mordon S, Sendid B, et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008;197(7):972–80. https://doi.org/10.1086/528990.
Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–7. https://doi.org/10.1126/science.1221789.
Koh AY. Identifying host immune effectors critical for protection against Candida albicans infections. Virulence. 2016;7(7):745–7. https://doi.org/10.1080/21505594.2016.1205177.
Koh AY. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell. 2013;12(11):1416–22. https://doi.org/10.1128/EC.00196-13.
Ahlawat S, De Jesus M, Khare K, Cole RA, Mantis NJ. Three-dimensional reconstruction of murine Peyer’s patches from immunostained cryosections. Microsc Microanal. 2014;20(1):198–205. https://doi.org/10.1017/S1431927613013640.
Singh N, Gallagher HC, Song R, Dhinsa JK, Ostroff GR, De Jesus M. RNA isolation from Peyer’s patch lymphocytes and mononuclear phagocytes to determine gene expression profiles using nanostring technology. J Biol Methods. 2018;5(3):e95. https://doi.org/10.14440/jbm.2018.246.
De Jesus M, Ostroff GR, Levitz SM, Bartling TR, Mantis NJ. A population of Langerin-positive dendritic cells in murine Peyer’s patches involved in sampling beta-glucan microparticles. PLoS ONE. 2014;9(3):e91002. https://doi.org/10.1371/journal.pone.0091002.
Goyer M, Loiselet A, Bon F, L’Ollivier C, Laue M, Holland G, et al. Intestinal cell tight junctions limit invasion of Candida albicans through active penetration and endocytosis in the early stages of the interaction of the fungus with the intestinal barrier. PLoS ONE. 2016;11(3):e0149159. https://doi.org/10.1371/journal.pone.0149159.
Rast TJ, Kullas AL, Southern PJ, Davis DA. Human epithelial cells discriminate between commensal and pathogenic interactions with Candida albicans. PLoS ONE. 2016;11(4):e0153165. https://doi.org/10.1371/journal.pone.0153165.
De Jesus M, Rodriguez AE, Yagita H, Ostroff GR, Mantis NJ. Sampling of Candida albicans and Candida tropicalis by Langerin-positive dendritic cells in mouse Peyer’s patches. Immunol Lett. 2015;168(1):64–72. https://doi.org/10.1016/j.imlet.2015.09.008.
Albac S, Schmitz A, Lopez-Alayon C, d’Enfert C, Sautour M, Ducreux A, et al. Candida albicans is able to use M cells as a portal of entry across the intestinal barrier in vitro. Cell Microbiol. 2016;18(2):195–210. https://doi.org/10.1111/cmi.12495.
Bonnardel J, Da Silva C, Masse M, Montanana-Sanchis F, Gorvel JP, Lelouard H. Gene expression profiling of the Peyer’s patch mononuclear phagocyte system. Genom Data. 2015;5:21–4. https://doi.org/10.1016/j.gdata.2015.05.002.
Maroilley T, Berri M, Lemonnier G, Esquerre D, Chevaleyre C, Melo S, et al. Immunome differences between porcine ileal and jejunal Peyer’s patches revealed by global transcriptome sequencing of gut-associated lymphoid tissues. Sci Rep. 2018;8(1):9077. https://doi.org/10.1038/s41598-018-27019-7.
Da Silva C, Wagner C, Bonnardel J, Gorvel JP, Lelouard H. The Peyer’s patch mononuclear phagocyte system at steady state and during infection. Front Immunol. 2017;8:1254. https://doi.org/10.3389/fimmu.2017.01254.
Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2(4):362–74. https://doi.org/10.1038/mi.2009.17.
Xu W, Solis NV, Filler SG, Mitchell AP. Pathogen gene expression profiling during infection using a nanostring ncounter platform. Methods Mol Biol. 2016;1361:57–65. https://doi.org/10.1007/978-1-4939-3079-1_3.
Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013;9(4):e1003315. https://doi.org/10.1371/journal.ppat.1003315.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. https://doi.org/10.1186/s13059-014-0550-8.
Chen J, Tambalo M, Barembaum M, Ranganathan R, Simoes-Costa M, Bronner ME, et al. A systems-level approach reveals new gene regulatory modules in the developing ear. Development. 2017;144(8):1531–43. https://doi.org/10.1242/dev.148494.
Ngo VL, Abo H, Maxim E, Harusato A, Geem D, Medina-Contreras O, et al. A cytokine network involving IL-36gamma, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc Natl Acad Sci USA. 2018;115(22):E5076–85. https://doi.org/10.1073/pnas.1718902115.
Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. https://doi.org/10.1038/nri3707.
Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37(10):2695–706. https://doi.org/10.1002/eji.200737409.
Wuyts W, Van Wesenbeeck L, Morales-Piga A, Ralston S, Hocking L, Vanhoenacker F, et al. Evaluation of the role of RANK and OPG genes in Paget’s disease of bone. Bone. 2001;28(1):104–7. https://doi.org/10.1016/S8756-3282(00)00411-7.
Nakamura Y, Kimura S, Hase K. M cell-dependent antigen uptake on follicle-associated epithelium for mucosal immune surveillance. Inflamm Regen. 2018;38:15. https://doi.org/10.1186/s41232-018-0072-y72.
Kanaya T, Ohno H. The mechanisms of M-cell differentiation. Biosci Microbiota Food Health. 2014;33(3):91–7. https://doi.org/10.12938/bmfh.33.912013-024.
Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183(9):5738–47. https://doi.org/10.4049/jimmunol.0901563jimmunol.0901563.
Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, et al. Immunodeficiencies. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349(6248):606–13. https://doi.org/10.1126/science.aaa4282.
Robinson RT, Huppler AR. The Goldilocks model of immune symbiosis with Mycobacteria and Candida colonizers. Cytokine. 2017;97:49–65. https://doi.org/10.1016/j.cyto.2017.05.015.
De Luca A, Montagnoli C, Zelante T, Bonifazi P, Bozza S, Moretti S, et al. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol. 2007;179(9):5999–6008.
Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):1005882. https://doi.org/10.1371/journal.ppat.1005882.
Cambi A, Gijzen K, de Vries IJ, Torensma R, Joosten B, Adema GJ, et al. An antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33(2):532–8. https://doi.org/10.1002/immu.200310029.
Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283(29):20590–9. https://doi.org/10.1074/jbc.M709334200M709334200.
Takahara K, Yashima Y, Omatsu Y, Yoshida H, Kimura Y, Kang YS, et al. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol. 2004;16(6):819–29. https://doi.org/10.1093/intimm/dxh084dxh084.
Gao N, Yu FS. Chitinase 3-Like 1 promotes Candida albicans killing and preserves corneal structure and function by controlling host antifungal responses. Infect Immun. 2015;83(10):4154–64. https://doi.org/10.1128/IAI.00980-15IAI.00980-15.
Low D, Subramaniam R, Lin L, Aomatsu T, Mizoguchi A, Ng A, et al. Chitinase 3-like 1 induces survival and proliferation of intestinal epithelial cells during chronic inflammation and colitis-associated cancer by regulating S100A9. Oncotarget. 2015;6(34):36535–50. https://doi.org/10.18632/oncotarget.54405440.
Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, Langen H, Thelen M, et al. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR41, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185(12):2171–6.
Williams EJ, Haque S, Banks C, Johnson P, Sarsfield P, Sheron N. Distribution of the interleukin-8 receptors, CXCR42 and CXCR42, in inflamed gut tissue. J Pathol. 2000;192(4):533–9.
Santoni G, Gismondi A, Liu JH, Punturieri A, Santoni A, Frati L, et al. Candida albicans expresses a fibronectin receptor antigenically related to alpha 5 beta 1 integrin. Microbiology. 1994;140(Pt11):2971–9. https://doi.org/10.1099/13500872-140-11-2971.
Tronchin G, Bouchara JP, Robert R, Senet JM. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect Immun. 1988;56(8):1987–93.
Alteber Z, Sharbi-Yunger A, Pevsner-Fischer M, Blat D, Roitman L, Tzehoval E, et al. The anti-inflammatory IFITM genes ameliorate colitis and partially protect from tumorigenesis by changing immunity and microbiota. Immunol Cell Biol. 2018;96(3):284–97. https://doi.org/10.1111/imcb.12000.
Gulati K, Gangele K, Agarwal N, Jamsandekar M, Kumar D, Poluri KM. Molecular cloning and biophysical characterization of CXCL3 chemokine. Int J Biol Macromol. 2018;107(Pt A):575–84. https://doi.org/10.1016/j.ijbiomac.2017.09.032.
Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol. 2017;46:9–20. https://doi.org/10.1016/j.exphem.2016.10.008.
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015;195(4):1341–9. https://doi.org/10.4049/jimmunol.1500861195/4/1341.
Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6(2):e1000661. https://doi.org/10.1371/journal.ppat.1000661.
Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JWM, Kullberg BJ. Endogenous interleukin (IL)–1α and IL-1β are crucial for host defense against disseminated Candidiasis. J Infect Dis. 2006;193(10):1419–26. https://doi.org/10.1086/503363.
Salvi V, Vermi W, Gianello V, Lonardi S, Gagliostro V, Naldini A, et al. Dendritic cell-derived VEGF-A plays a role in inflammatory angiogenesis of human secondary lymphoid organs and is driven by the coordinated activation of multiple transcription factors. Oncotarget. 2016;7(26):39256–69. https://doi.org/10.18632/oncotarget.96849684.
Laurent S, Carrega P, Saverino D, Piccioli P, Camoriano M, Morabito A, et al. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum Immunol. 2010;71(10):934–41. https://doi.org/10.1016/j.humimm.2010.07.007.
Guimbaud R, Abitbol V, Bertrand V, Quartier G, Chauvelot-Moachon L, Giroud J, et al. Leukemia inhibitory factor involvement in human ulcerative colitis and its potential role in malignant course. Eur Cytokine Netw. 1998;9(4):607–12.
Smeekens SP, van de Veerdonk FL, Joosten LA, Jacobs L, Jansen T, Williams DL, et al. The classical CD14(+)(+) CD16(-) monocytes, but not the patrolling CD14(+) CD16(+) monocytes, promote Th17 responses to Candida albicans. Eur J Immunol. 2011;41(10):2915–24. https://doi.org/10.1002/eji.201141418.
Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46(7):503–12.
Meri T, Blom AM, Hartmann A, Lenk D, Meri S, Zipfel PF. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect Immun. 2004;72(11):6633–41. https://doi.org/10.1128/IAI.72.11.6633-6641.2004.
Rambach G, Speth C. Complement in Candida albicans infections. Front Biosci (Elite Ed). 2009;1:1–12.
Fordham JB, Hua J, Morwood SR, Schewitz-Bowers LP, Copland DA, Dick AD, et al. Environmental conditioning in the control of macrophage thrombospondin-1 production. Sci Rep. 2012;2:512. https://doi.org/10.1038/srep00512.
Martin-Manso G, Navarathna DH, Galli S, Soto-Pantoja DR, Kuznetsova SA, Tsokos M, et al. Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis. PLoS ONE. 2012;7(11):e48775. https://doi.org/10.1371/journal.pone.0048775.
Yi YS. Functional role of milk fat globule-epidermal growth factor VIII in macrophage-mediated inflammatory responses and inflammatory/autoimmune diseases. Mediators Inflamm. 2016;2016:5628486. https://doi.org/10.1155/2016/5628486.
Chogle A, Bu HF, Wang X, Brown JB, Chou PM, Tan XD. Milk fat globule-EGF factor 8 is a critical protein for healing of dextran sodium sulfate-induced acute colitis in mice. Mol Med. 2011;17(5–6):502–7. https://doi.org/10.2119/molmed.2010.00074.
Gaffen SL, Hernandez-Santos N, Peterson AC. IL-17 signaling in host defense against Candida albicans. Immunol Res. 2011;50(2–3):181–7. https://doi.org/10.1007/s12026-011-8226-x.
Conti HR, Gaffen SL. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 2015;195(3):780–8. https://doi.org/10.4049/jimmunol.1500909.
Chin VK, Foong KJ, Maha A, Rusliza B, Norhafizah M, Chong PP. Early expression of local cytokines during systemic Candida albicans infection in a murine intravenous challenge model. Biomed Rep. 2014;2(6):869–74.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. https://doi.org/10.3389/fimmu.2014.00491.
van Enckevort FH, Netea MG, Hermus AR, Sweep CG, Meis JF, Van der Meer JW, et al. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice. Med Mycol. 1999;37(6):419–26.
Triebel T, Grillhosl B, Kacani L, Lell CP, Fuchs A, Speth C, et al. Importance of the terminal complement components for immune defence against Candida. Int J Med Microbiol. 2003;292(7–8):527–36. https://doi.org/10.1078/1438-4221-00211.
Wang S, Song R, Wang Z, Jing Z, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. https://doi.org/10.3389/fimmu.2018.01298.
Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PL Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE. 2012;7(9):e46311. https://doi.org/10.1371/journal.pone.0046311.
Yano J, Lilly E, Barousse M, Fidel PL Jr. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78(12):5126–37. https://doi.org/10.1128/IAI.00388-10IAI.00388-10.
Yano J, Noverr MC, Fidel PL Jr. Cytokines in the host response to Candida vaginitis: identifying a role for non-classical immune mediators, S100 alarmins. Cytokine. 2012;58(1):118–28. https://doi.org/10.1016/j.cyto.2011.11.021S1043-4666(11)00831-3.
Bjerknes R, Aarskog D. Priming of human polymorphonuclear neutrophilic leukocytes by insulin-like growth factor I: increased phagocytic capacity, complement receptor expression, degranulation, and oxidative burst. J Clin Endocrinol Metab. 1995;80(6):1948–55. https://doi.org/10.1210/jcem.80.6.7775645.
Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37(3):463–74. https://doi.org/10.1016/j.immuni.2012.06.012.
Vulcano M, Albanesi C, Stoppacciaro A, Bagnati R, D’Amico G, Struyf S, et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol. 2001;31(3):812–22. https://doi.org/10.1002/1521-4141(200103)31:3%3c812:AID-IMMU812%3e3.0.CO;2-L.
Yun B, Lee H, Jayaraja S, Suram S, Murphy RC, Leslie CC. Prostaglandins from cytosolic phospholipase A2alpha/cyclooxygenase-1 pathway and mitogen-activated protein kinases regulate gene expression in Candida albicans-infected macrophages. J Biol Chem. 2016;291(13):7070–86. https://doi.org/10.1074/jbc.M116.714873M116.714873.
Yuan X, Hua X, Wilhelmus KR. Proinflammatory chemokines during Candida albicans keratitis. Exp Eye Res. 2010;90(3):413–9. https://doi.org/10.1016/j.exer.2009.12.001.
Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM, Brown GD. Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect Immun. 2012;80(12):4216–22. https://doi.org/10.1128/IAI.00559-12.
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252–66. https://doi.org/10.1016/j.clim.2003.11.017.
Castillo L, MacCallum DM. Cytokine measurement using cytometric bead arrays. Methods Mol Biol. 2012;845:425–34. https://doi.org/10.1007/978-1-61779-539-8_29.
Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247. https://doi.org/10.3389/fmicb.2018.02247.
Xiao L, Feng Q, Liang S, Sonne SB, Xia Z, Qiu X, et al. A catalog of the mouse gut metagenome. Nat Biotechnol. 2015;33(10):1103–8. https://doi.org/10.1038/nbt.3353.
Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14(6):405–16. https://doi.org/10.1038/nri3684.
Soll DR, Galask R, Schmid J, Hanna C, Mac K, Morrow B. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J Clin Microbiol. 1991;29(8):1702–10.
Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21(7):334–41. https://doi.org/10.1016/j.tim.2013.04.002.
Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G518–25. https://doi.org/10.1152/ajpgi.00024.2006.
Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2(4):362–74. https://doi.org/10.1038/mi.2009.17mi200917.
Drakes ML, Lu L, Mckenna HJ, Thomson AW. The influence of collagen, fibronectin, and laminin on the maturation of dendritic cell progenitors propagated from normal or Flt3-ligand-treated mouse liver. Boston: Springer; 1997.
Acknowledgements
We thank the Wadsworth Center Applied Genomic Technologies Core and the Wadsworth Center Biochemistry and Immunology Core. We thank Dr. Kelly Miller of Nanostring Technologies for assistance with technical support and data analysis. We thank Yannick David for his help in generating the PP model. We thank Dr. Sudha Chaturvedi at the Wadsworth Center Mycology laboratory for providing C. albicans strain ATCC 18804. We thank Dr. Aaron Mitchell at Carnegie Mellon University for providing C. albicans strain SC5314. We thank Dr. Kimberly McClive-Reed of Health Research, Inc. for critical reading of this manuscript. M.D.J. was supported by the University at Albany and Wadsworth Center start-up funds. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Ferry Hagen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, N., Kim, H.C., Song, R. et al. Candida albicans Elicits Pro-Inflammatory Differential Gene Expression in Intestinal Peyer’s Patches. Mycopathologia 184, 461–478 (2019). https://doi.org/10.1007/s11046-019-00349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-019-00349-4