Abstract
Purpose
Vulvovaginal candidiasis (VVC) is a common superficial infection of the vaginal mucous membranes caused by the fungus Candida albicans. The aim of this study was to assess the mechanisms underlying the inhibitory effects of the culture supernatants of Lactobacillus gasseri and L. crispatus, the predominant microbiota in Asian healthy women, on C. albicans biofilm formation. The inhibition of C. albicans adhesion to HeLa cells by Lactobacillus culture supernatant was also investigated.
Methods
Candida albicans biofilm was formed on polystyrene flat-bottomed 96-well plates, and the inhibitory effects on the initial colonization and maturation phases were determined using the XTT reduction assay. The expression levels of biofilm formation-associated genes (HWP1, ECE1, ALS3, BCR1, EFG1, TEC1, and CPH1) were determined by reverse transcription quantitative polymerase chain reaction. The inhibition of C. albicans adhesion to HeLa cells by Lactobacillus culture supernatant was evaluated by enumerating viable C. albicans cells.
Results
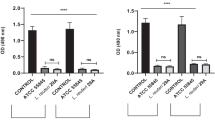
The culture supernatants of both Lactobacillus species inhibited the initial colonization and maturation of C. albicans biofilm. The expression levels of all biofilm formation-related genes were downregulated in the presence of Lactobacillus culture supernatant. The culture supernatant also inhibited C. albicans adhesion to HeLa cells.
Conclusion
The culture supernatants of L. gasseri and L. crispatus inhibited C. albicans biofilm formation by downregulating biofilm formation-related genes and C. albicans adhesion to HeLa cells. These findings support the notion that Lactobacillus metabolites may be useful alternatives to antifungal drugs for the management of VVC.





Similar content being viewed by others
References
Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep. 2015;17:462. https://doi.org/10.1007/s11908-015-0462-0.
Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266–72. https://doi.org/10.1093/jac/dkl246.
Chew SY, Cheah YK, Seow HF, Sandai D, Than LT. In vitro modulation of probiotic bacteria on the biofilm of Candida glabrata. Anaerobe. 2015;34:132–8. https://doi.org/10.1016/j.anaerobe.2015.05.009.
Zhou X, Westman R, Hickey R, Hansmann MA, Kennedy C, Osborn TW, Forney LJ. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect Immun. 2009;77:4130–5. https://doi.org/10.1128/IAI.00436-09.
Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–76. https://doi.org/10.1128/MMBR.00009-06.
Midkiff J, Borochoff-Porte N, White D, Johnson DI. Small molecule inhibitors of the Candida albicans budded-to-hyphal transition act through multiple signaling pathways. PLoS ONE. 2011;6:e25395. https://doi.org/10.1371/journal.pone.0025395.
Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9:737–48. https://doi.org/10.1038/nrmicro2636.
Matsubara VH, Wang Y, Bandara HM, Mayer MP, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl Microbiol Biotechnol. 2016;100:6415–26. https://doi.org/10.1007/s00253-016-7527-3.
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108:4680–7. https://doi.org/10.1073/pnas.1002611107.
Anderson BL, Mendez-Figueroa H, Dahlke JD, Raker C, Hillier SL, Cu-Uvin S. Pregnancy-induced changes in immune protection of the genital tract: defining normal. Am J Obstet Gynecol. 2013;208:321.e1-9. https://doi.org/10.1016/j.ajog.2013.01.014.
Liu MB, Xu SR, He Y, Deng GH, Sheng HF, Huang XM, Ouyang CY, Zhou HW. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS ONE. 2013;8:e79812. https://doi.org/10.1371/journal.pone.0079812.
Morales DK, Hogan DA. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010;6:e1000886. https://doi.org/10.1371/journal.ppat.1000886.
Ribeiro FC, de Barros PP, Rossoni RD, Junqueira JC, Jorge AO. Lactobacillus rhamnosus inhibits Candida albicans virulence factors in vitro modulates immune system in Galleria mellonella. J Appl Microbiol. 2017;122:201–11. https://doi.org/10.1111/jam.13324.
Nett JE, Cain MT, Crawford K, Andes DR. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J Clin Microbiol. 2011;49:1426–33. https://doi.org/10.1128/JCM.02273-10.
Kurakado S, Takatori K, Sugita T. Minocycline inhibits the Candida albicans budded-to-hyphal-form transition and biofilm formation. Jpn J Infect Dis. 2017;70:490–4. https://doi.org/10.7883/yoken.JJID.2016.369.
Wang S, Wang Q, Yang E, Yan L, Li T, Zhuang H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front Microbiol. 2017;8:564. https://doi.org/10.3389/fmicb.2017.00564.
Coman MM, Verdenelli MC, Cecchini C, Silvi S, Orpianesi C, Caspani M, Mondello F, Cresci A. In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J Appl Microbiol. 2015;119:1383–90. https://doi.org/10.1111/jam.12947.
Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9:109–18. https://doi.org/10.1038/nrmicro2475.
Desai JV, Mitchell AP. Candida albicans biofilm development and its genetic control. Microbiol Spectr. 2015. https://doi.org/10.1128/microbiolspec.MB-0005-2014.
Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol. 2006;48:424–32. https://doi.org/10.1111/j.1574-695X.2006.00162.x.
Kang CH, Han SH, Kim Y, Paek NS, So JS. In vitro probiotic properties of Lactobacillus salivarius MG242 isolated from human vagina. Probiotics Antimicrob Proteins. 2017. https://doi.org/10.1007/s12602-017-9323-5.
Verdenelli MC, Coman MM, Cecchini C, Silvi S, Orpianesi C, Cresci A. Evaluation of antipathogenic activity and adherence properties of human Lactobacillus strains for vaginal formulations. J Appl Microbiol. 2014;116:1297–307. https://doi.org/10.1111/jam.12459.
Parolin C, Marangoni A, Laghi L, Foschi C, Ñahui Palomino RA, Calonghi N, Cevenini R, Vitali B. Isolation of vaginal lactobacilli and characterization of anti-Candida activity. PLoS ONE. 2015;10:e0131220. https://doi.org/10.1371/journal.pone.0131220.
Harriott MM, Noverr MC. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19:557–63. https://doi.org/10.1016/j.tim.2011.07.004.
James KM, MacDonald KW, Chanyi RM, Cadieux PA, Burton JP. Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J Med Microbiol. 2016;65:328–36. https://doi.org/10.1099/jmm.0.000226.
Calonghi N, Parolin C, Sartor G, Verardi L, Giordani B, Frisco G, Marangoni A, Vitali B. Interaction of vaginal Lactobacillus strains with HeLa cells plasma membrane. Benefic Microbes. 2017;8:625–33. https://doi.org/10.3920/BM2016.0212.
Cribby S, Taylor M, Reid G. Vaginal microbiota and the use of probiotics. Interdiscip Perspect Infect Dis. 2008;2008:256490. https://doi.org/10.1155/2008/256490.
Samaranayake YH, Cheung BPK, Yau JYY, Yeung SKW, Samaranayake LP. Human serum promotes Candida albicans biofilm growth and virulence gene expression on silicone biomaterial. PLoS ONE. 2013;8:e62902. https://doi.org/10.1371/journal.pone.0062902.
Bassilana M, Hopkins J, Arkowitz RA. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot Cell. 2005;4:588–603. https://doi.org/10.1128/EC.4.3.588-603.2005.
Uppuluri P, Chaturvedi AK, Lopez-Ribot JL. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia. 2009;168:101–9. https://doi.org/10.1007/s11046-009-9205-9.
Hierro N, Esteve-Zarzoso B, González A, Mas A, Guillamón JM. Real-time quantitative PCR (QPCR) and reverse transcription-QPCR for detection and enumeration of total yeasts in wine. Appl Environ Microbiol. 2006;72:7148–55. https://doi.org/10.1128/AEM.00388-06.
Acknowledgements
This study was supported in part by the Research Program on Emerging and Re-emerging Infectious Diseases of the Japan Agency for Medical Research and Development, and the Japan Society for the Promotion of Science, KAKENHI (to TS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Vishnu Chaturvedi.
Rights and permissions
About this article
Cite this article
Matsuda, Y., Cho, O., Sugita, T. et al. Culture Supernatants of Lactobacillus gasseri and L. crispatus Inhibit Candida albicans Biofilm Formation and Adhesion to HeLa Cells. Mycopathologia 183, 691–700 (2018). https://doi.org/10.1007/s11046-018-0259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-018-0259-4