Abstract
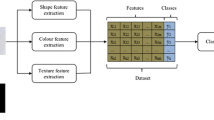
Melanoma is a skin disorder, occurring in melanocytes. They are classified as Benign and Malignant. The cure of melanoma is effective, if it can be recognized early. The most crucial part in the cure of melanoma is the exact classification and determining the group of melanoma. A comparative study for classifying the group of melanoma using the supervised machine learning algorithms is discussed in this proposed work. Classification of melanoma from dermoscopic data is proposed to help the clinical utilization of dermatoscopy imaging methods for skin sores classification. The images were enhanced using anisotropic diffusion filter and unsharp masking. The melanoma was segmented from the background using adaptive k-means clustering algorithm with two clusters followed by feature extraction methods are based on intensity and texture features from the segmented data, which is followed by training of classifier and finally testing on unknown dermoscopic data. Classifiers such as k-nearest neighbour, support vector machine, multi-layer perceptron, decision tree and random forest were used. To test the performance of the classifiers, the area under the receiver operating characteristics curve (ROC) is utilized. The Random forest method is found to achieve 93% accuracy and classifies melanoma significantly good as compared to other classifiers.





Similar content being viewed by others
References
Al-azawi RJ, Abdulhameed AA, Ahmed HM (2017) A robustness segmentation approach for skin Cancer image detection based on an adaptive automatic thresholding technique. Am J Intell Syst 7:107–112
Andre E, Brett K, Novoa Roberto A, Justin K, Swetter Susan M, Blau Helen M, Sebastian T (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542:115–118
Bezdek JC, Ehrlich R, Full W (1984) FCM: the fuzzy c-means clustering algorithm. Comput Geosci 10:191–203
Bhatia SK (2004) Adaptive k-means clustering. FLAIRS Conf Am Assoc Artif Intell: 695–699
Breiman, L. (2001) Random forests. Machine Learning, pp. 5–32, Springer
Codella NCF, Gutman D, Celebi ME, Helba B, Marchetti MA, Dusza SW et al. (2017) Skin lesion analysis toward melanoma detection: A challenge at the 2017 international symposium on biomedical imaging. Int Skin Imag Collab (ISIC). arXiv Prepr arXiv171005006
Cunningham P, Delany SJ (2007) k-Nearest neighbour classifiers. Mult Classif Syst 34:1–17
Ebtihal A, Arfan JM (2016) Classification of Dermoscopic skin cancer images using color and hybrid texture features. Int J Comput Sci Netw Secur 16(4):135–139
Eltayef K, Li Y, Liu X (2017) Detection of melanoma skin cancer in dermoscopy images. J Phys Conf Ser 787:12034–12041
Falcidieno B, Giannini F (1989) Automatic recognition and representation of shape-based features in a geometric modeling system. Comput Vision Graph Image Process 48:93–123
Feng Y, Kawrakow I, Olsen J, Parikh PJ (2016) A comparative study of automatic image segmentation algorithms for target tracking in MR-IGRT. J Appl Clin Med Phys 17(2):441–460
Gautam D, Ahmed M, Meena YK, Ul HA (2018) Machine learning-based diagnosis of melanoma using macro images. Int J Numer Method Biomed Eng 34(5):e2953. https://doi.org/10.1002/cnm.2953
Gershenwald JE, Scolyer RA, Hess KR (2017) Melanoma staging: evidence-based changes in the American joint committee on Cancer eighth edition. Cancer Staging Manual, Cancer J Clin 67(6):474–492
Guo Z, Zhang L, Zhang DA (2010) Completed modeling of local binary pattern operator for texture classification. IEEE Trans Image Process 19:1657–16563
Hartigan JA, Wong MA (1979) Algorithm AS 136: A k-means clustering algorithm. J R Stat Soc Ser C (Applied Stat) 28(1):100–108
Hu P, Yang T (2016) Pigmented skin lesion detection using random forest and wavelet-based texture. Proc SPIE. https://doi.org/10.1117/12.2245149
ISIC (2016) ISIC Archieve : The International Skin Imaging Collaboration: Melanoma Project," ISIC. [Online]. Available: https://isic-archive.com/#. [Accessed 20 Jan 2018]
Jain S, Jagtap V, Pise N (2015) Computer aided melanoma skin cancer detection using image processing. Proc Comput Sci 48:736–741
Jaisakthi SM, Chandrabose A, Mirunalini P (2017) Automatic skin lesion segmentation using semi-supervised learning technique. Comput Vision Pattern Recogn. arXiv preprint arXiv:1703.04301
Khalid S, Jamil U, Saleem K, Akram MU, Manzoor W, Ahmed W et al (2016) Segmentation of skin lesion using Cohen–Daubechies–Feauveau biorthogonal wavelet. Springerplus. https://doi.org/10.1186/s40064-016-3211-4
Li Y, Shen L (2018) Skin lesion analysis towards melanoma detection using deep learning network. Sensors (Basel) 18(2):556. https://doi.org/10.3390/s18020556
Lu C, Mandal M (2015) Automated analysis and diagnosis of skin melanoma on whole slide histopathological images. Pattern Recogn 48:2738–2750
Mohd A, Ram GK, Shafeeq A (2017) Skin cancer classification using K-means clustering. Int J Tech Res Appl 5(1):62–65
Nasir M, Khan MA, Sharif M, Lali IU, Saba T, Iqbal T (2018) An improved strategy for skin lesion detection and classification using uniform segmentation and feature selection based approach. Micros Res Tech 81(6):528–543
National Toxicology Program (2002) Ultraviolet radiation related exposures: broad-spectrum ultraviolet (UV) radiation, UVA, UVB, UVC, solar radiation, and exposure to sunlamps and sunbeds. Rep Carcinog Carcinog Profiles 10:250–254
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9:62–66
Paja W, Wrzesien M (2013) Melanoma important features selection using random forest approach. 6th Int Conf Hum Syst Interact HSI. https://doi.org/10.1109/HSI.2013.6577857
Pennisi A, Bloisi DD, Nardi D, Giampetruzzi AR, Mondino C, Facchiano A (2016) Skin lesion image segmentation using Delaunay triangulation for melanoma detection. Comput Med Imaging Graph 52:89–103
Perona P, Malik J (1990) Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell 12:629–639
Polesel A, Ramponi G, Mathews VJ (2000) Image enhancement via adaptive unsharp masking. IEEE Trans Image Process 9:505–510
Rofman D, Hart G, Girardi M, Ko CJ, Deng J (2018) Predicting non-melanoma skin cancer via a multi-parameterized artifcial neural network. Nature 8(1701):1–7
Ruck DW, Rogers SK, Kabrisky M, Oxley ME, Suter BW (1990) The multilayer perceptron as an approximation to a Bayes optimal discriminant function. IEEE Trans Neural Netw 1:296–298
Rundo F, Conoci S, Petralia S, Banna GL, Rundo F (2017) Advanced bio-inspired point of care for skin cancer early detection. SL Clin Med Oncol 1(1):111–116
Safavian SR, Landgrebe D (1991) A survey of decision tree classifier methodology. IEEE Trans Syst Man Cybern 21:660–674
Salerni G, Terán T, Puig S, Malvehy J, Zalaudek I, Argenziano G et al (2013) Meta analysis of digital dermoscopy follow up of melanocytic skin lesions: a study on behalf of the international Dermoscopy society. J Eur Acad Dermatology Venereol 27:805–814
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics 2018. CA Cancer J Clin 68(1):7–30
Soh LK, Tsatsoulis C (1999) Texture analysis of SAR Sea ice imagery using gray level co-occurrence matrices. IEEE Trans Geosci Remote Sens 37:780–795
Suykens JAK, Vandewalle J (1999) Least squares support vector machine classifiers. Neural Process Lett 9:293–300
Taha AA, Hanbury A (2015) Metrics for evaluating 3D medical image segmentation: analysis, selection, and tool. BMC Med Imaging 15:–29. https://doi.org/10.1186/s12880-015-0068-x
Telea A (2004) An image inpainting technique based on the fast marching method. J Graph Tools 9:23–34
Van De Weijer J, Schmid C (2006) Coloring local feature extraction. Eur Conf Comput Vis: 334–48. Springer
Victor A, Ghalib MR (2017) A hybrid segmentation approach for detection and classification of skin cancer. Biomed Res 28(16):6947–6954
Wesley, JChun.: . Core python programming. Prentice hall professional, United States of America ( 2006)
Zakeri A, Hokmabadi A (2018) Improvement in the diagnosis of melanoma and dysplastic lesions by introducing ABCD-PDT features and a hybrid classifier. Biocybern Biomed Eng. https://doi.org/10.1016/j.bbe.2018.03.005
Zhang H, Fritts JE, Goldman SA (2008) Image segmentation evaluation: a survey of unsupervised methods. Comput Vis Image Und 110(2):260–280
Zou KH, Warfield SK, Bharatha A, Tempany CMC, Kaus MR, Haker SJ et al (2004) Statistical validation of image segmentation quality based on a spatial overlap index1. scientific reports. Acad Radiol 11:178–189
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical clearance
Since, the data was collected from a publically available online database; there was no requirement of ethical clearance from institutional review board (IRB).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Janney.J, B., Roslin, S. Classification of melanoma from Dermoscopic data using machine learning techniques. Multimed Tools Appl 79, 3713–3728 (2020). https://doi.org/10.1007/s11042-018-6927-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-018-6927-z