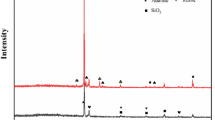
An economical Al – Cu powder with high conductivity has been synthesized by a chemical substitution reaction. The powder particles have the form of an aluminium core, on which a copper shell is deposited. The deposition reaction occurs in a copper sulphate solution at a temperature of 45°C. At higher temperatures, copper diffusion proceeds faster, but the aluminium core may crack. The process of aluminium substitution with copper on the particle surface develops at different values of the standard potential of each metal. The minimum resistivity of 1.01 × 10 – 7 Ω ∙ m is obtained in a composite sintered at 550°C from the Al-core Cu-shell powder with addition of 3 wt.% glass frit. This material can find wide application, for example, as a substitute for silver in electronic devices to reduce production costs.






Similar content being viewed by others
References
G. Li, X. X. Huang, and J. K. Guo, “Fabrication and mechanical properties of Al2O3 – Ni composite from two different powder mixtures,” Mater. Sci. Eng. A, 352(1 – 2), 23 – 28 (2003).
T. Puclin and W. A. Kaczmarek, “Synthesis of alumina-nitride nanocomposites by successive reduction-nitridation in mechanochemically activated reactions,” J. Alloy. Compd., 266(1 – 2), 283 – 292 (1998).
G. M. Shi, J. K. Han, Z. D. Zhang, et al., “Pretreatment effect on the synthesis of Ag-coated Al2O3 powders by electroless deposition process,” Surf. Coat. Technol., 195(2 – 3), 333 – 337 (2005).
V. V. Srdić, B. Mojić, M. Nikolić, and S. Ognjanović, “Recent progress on synthesis of ceramics core/shell nanostructures,” Process. Appl. Ceram., 7(2), 45 – 62 (2013).
T. Ji, V. G. Lirtsman, Y. Avny, and D. Davidov, “Preparation, characterization, and application of Au-shell/polystyrene beads and Au-shell_magnetic beads,” Adv. Mater., 13(16), 1253 – 1256 (2001).
J. Chen, B. Wiley, J. McLellan, et al., “Optical properties of Pd – Ag and Pt – Ag nanoboxes synthesized via galvanic replacement reactions,” Nano Lett., 5(10), 2058 – 2062 (2005).
W. Stöber, A. Fink, and E. Bohn, “Controlled growth of monodisperse silica spheres in the micron size range,” J. Colloid. Interf. Sci., 26(1), 62 – 69 (1968).
N. R. Jana, C. Earhart, and J. Y. Ying, “Synthesis of water-soluble and functionalized nanoparticles by silica coating,” Chem. Mater., 19(21), 5074 – 5082 (2007).
D. V. Matyushov, “Standard electrode potential, Tafel equation, and the solvation thermodynamics,” J. Chem. Phys., 130(23), 234704 (2009).
K. C. Chuang andW. H. Lee, “Improvement on conductivity for thick film aluminum paste,” J. Nanosci. Nanotechnol., 21(9), 4726 – 4734 (2021).
J. Li, J. W. Mayer, and E. G. Colgan, “Oxidation and protection in copper and copper alloy thin films,” J. Appl. Phys., 70(5), 2820 – 2827 (1991).
C. Xu, Y. Liu, J. Wang, et al., “Fabrication of nanoporous Cu – Pt(Pd) core_shell structure by galvanic replacement and its application in electrocatalysis,” ACS Appl. Mater. Interf., 3(12), 4626 – 4632 (2011).
T. Gustmann, J. M. dos Santos, P. Gargarella, et al., “Properties of Cu-based shape-memory alloys prepared by selective laser melting,” Shap. Mem. Superelasticity, 3(1), 24 – 36 (2017).
S. Zeller and J. Gnauk, “Shape memory behavior of Cu – Al wires produced by horizontal in-rotating-liquid-spinning,” Mater. Sci. Eng. A, 481, 562 – 566 (2008).
S. Yu. Kondrat’ev and O. V. Shvetsov, “Effect of high-temperature heating on the structure and properties of aluminum alloys in the production of drill pipes,” Met. Sci. Heat Treat., 55(3 – 4), 191 – 196 (2013).
A. T. Adorno and R. A. G. Silva, “Ageing behavior in the Cu – 10 wt.% Al and Cu – 10 wt.% Al – 4 wt.% Ag alloys,” J. Alloy. Compd., 473(1 – 2), 139 – 144 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 6, pp. 65 – 72, June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puteri, N.G., Yu, Y.W. & Lee, W.H. Increasing the Conductivity of Sintered Powder Al – Cu Material Due to Electroplating of Aluminium Particles with Copper. Met Sci Heat Treat 65, 386–392 (2023). https://doi.org/10.1007/s11041-023-00942-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-023-00942-x