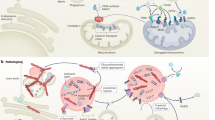
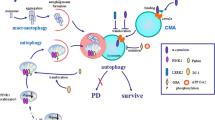
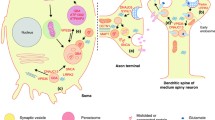
Abstract
Parkinson’s disease (PD) is a complex and debilitating neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra. The pathogenesis of PD is intimately linked to the roles of two key molecular players, α-synuclein (α-syn) and Parkin. Understanding the intricate interplay between α-syn and Parkin is essential for unravelling the molecular underpinnings of PD. Their roles in synaptic function and protein quality control underscore their significance in neuronal health. Dysregulation of these processes, as seen in PD, highlights the potential for targeted therapeutic strategies aimed at restoring normal protein homeostasis and mitigating neurodegeneration. Investigating the connections between α-syn, Parkin, and various pathological mechanisms provides insights into the complex web of factors contributing to PD pathogenesis and offers hope for the development of more effective treatments for this devastating neurological disorder. The present compilation provides an overview of their structures, regional and cellular locations, associations, physiological functions, and pathological roles in the context of PD.
Graphical abstract




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AFM:
-
Atomic Force Microscopy
- CD:
-
circular dichroism
- DA:
-
Dopamine
- DAT:
-
Dopamine Transporter
- ER:
-
Endoplasmic Reticulum
- FRET:
-
fluorescence resonance energy transfer
- GSK-3β:
-
Glycogen Synthase Kinase-3β
- IBR:
-
In-Between RING
- iNOS:
-
inducible Nitric Oxide Synthase
- NAC:
-
non- amyloid -β component
- NF-kB:
-
Nuclear Factor-kappa B NMDA, N-methyl-D-aspartate
- PD:
-
Parkinson’s disease
- PD2:
-
Phospholipase D2
- RBR:
-
RING-between-RING
- ROS:
-
Reactive Oxygen Species
- SN:
-
Subtantia nigra
- SNARE:
-
Soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors
- TGN:
-
trans-Golgi network
- TH:
-
Tyrosine Hydroxylase
- TNF-α:
-
Tumor Necrotic Factor-α
- VMAT:
-
Vesicular Monoamine Transporter
- UPS:
-
Ubiquitin Proteasomal Sysytem
References
Bartels AL, Leenders KL (2009) Parkinson’s disease: the syndrome, the pathogenesis and pathophysiology. Cortex 45(8):915–921
Moustafa AA et al (2016) Motor symptoms in Parkinson’s disease: a unified framework. Neurosci Biobehav Rev 68:727–740
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18(7):435–450
Kawahara K et al (2008) Alpha-synuclein aggregates interfere with Parkin solubility and distribution: role in the pathogenesis of Parkinson disease. J Biol Chem 283(11):6979–6987
Yoo G, Shin YK, Lee NK (2023) The role of α-Synuclein in SNARE-mediated synaptic Vesicle Fusion. J Mol Biol 435(1):167775
Manna M, Murarka RK (2021) Polyunsaturated fatty acid modulates membrane-bound Monomeric α-Synuclein by modulating membrane microenvironment through preferential interactions. ACS Chem Neurosci 12(4):675–688
Lesage S et al (2013) G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol 73(4):459–471
Lunati A, Lesage S, Brice A (2018) The genetic landscape of Parkinson’s disease. Rev Neurol (Paris) 174(9):628–643
Correia Guedes L et al (2020) Are genetic and idiopathic forms of Parkinson’s disease the same disease? J Neurochem 152(5):515–522
Perfeito R, Cunha-Oliveira T, Rego AC (2013) Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med 62:186–201
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci 30(5):244–250
Goedert M (1997) The awakening of α-synuclein. Nature 388(6639):232–233
Breydo L, Wu JW, Uversky VN (2012) Α-synuclein misfolding and Parkinson’s disease Biochim Biophys Acta, 1822(2): pp. 261 – 85
Bartels T, G Choi J, J Selkoe D (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477(7362):107–110
Ghosh D et al (2015) Structure based aggregation studies reveal the presence of helix-rich intermediate during α-Synuclein aggregation. Sci Rep 5(1):9228
Giasson BI et al (2001) A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 276(4):2380–2386
Stephens AD, Zacharopoulou M (2019) K.J.T.i.b.s. Schierle. Cell Environ Affects Monomeric α-synuclein Struct 44(5):453–466
Halliday GM, McCann H (2008) Human-based studies on alpha-synuclein deposition and relationship to Parkinson’s disease symptoms. Exp Neurol 209(1):12–21
Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8(8):2804–2815
Zhong SC et al (2010) Expression and subcellular location of alpha-synuclein during mouse-embryonic development. Cell Mol Neurobiol 30(3):469–482
Giasson BI et al (2001) Prominent perikaryal expression of alpha- and beta-synuclein in neurons of dorsal root ganglion and in medullary neurons. Exp Neurol 172(2):354–362
Polymeropoulos MH et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276(5321):2045–2047
Calo L et al (2016) Synaptic failure and α-synuclein. Mov Disord 31(2):169–177
El-Agnaf OM et al (2003) Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. Faseb j 17(13):1945–1947
Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25(25):6016–6024
McLean PJ et al (2000) Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson’s disease-linked mutations. J Biol Chem 275(12):8812–8816
Cheng F, Vivacqua G, Yu S (2011) The role of α-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat 42(4):242–248
Masliah E et al (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287(5456):1265–1269
Kirik D et al (2002) Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 22(7):2780–2791
Lotharius J et al (2002) Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem 277(41):38884–38894
Gadhavi J et al (2022) Neurotoxic or neuroprotective: post-translational modifications of α-synuclein at the cross-roads of functions. Biochimie 192:38–50
Mizuno Y et al (2001) Parkin and Parkinson’s disease. Curr Opin Neurol 14(4):477–482
Fakih R, Sauvé V, Gehring K (2022) Structure of the second phosphoubiquitin-binding site in parkin. J Biol Chem 298(7):102114
Beasley SA, Hristova VA, Shaw GS (2007) Structure of the parkin in-between-ring domain provides insights for E3-ligase dysfunction in autosomal recessive Parkinson’s disease. Proc Natl Acad Sci U S A 104(9):3095–3100
Seirafi M, Kozlov G, Gehring K (2015) Parkin structure and function. Febs j 282(11):2076–2088
van der Reijden BA et al (1999) TRIADs: a new class of proteins with a novel cysteine-rich signature. Protein Sci 8(7):1557–1561
Imai Y, Soda M, Takahashi R (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem 275(46):35661–35664
Kubo SI et al (2001) Parkin is associated with cellular vesicles. J Neurochem 78(1):42–54
Horowitz JM et al (1999) Identification and distribution of Parkin in rat brain. NeuroReport 10(16):3393–3397
Horowitz JM et al (2001) Immunodetection of Parkin protein in vertebrate and invertebrate brains: a comparative study using specific antibodies. J Chem Neuroanat 21(1):75–93
Kitada T et al (2000) Molecular cloning, gene expression, and identification of a splicing variant of the mouse parkin gene. Mamm Genome 11(6):417–421
Kühn K et al (2004) Parkin expression in the developing mouse. Brain Res Dev Brain Res 149(2):131–142
Ledesma MD et al (2002) Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J Neurochem 83(6):1431–1440
Sassone J et al (2017) The synaptic function of parkin. Brain 140(9):2265–2272
Jiang H et al (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 3(1):668
Maraschi A et al (2014) Parkin regulates kainate receptors by interacting with the GluK2 subunit. Nat Commun 5:5182
Palacino JJ et al (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279(18):18614–18622
Sarraf SA et al (2019) PINK1/Parkin influences cell cycle by sequestering TBK1 at Damaged Mitochondria, inhibiting mitosis. Cell Rep 29(1):225–235e5
Takahashi R et al (2003) Parkin and endoplasmic reticulum stress. Ann N Y Acad Sci 991:101–106
Storz G, Imlay JA (1999) Oxidative stress. Curr Opin Microbiol 2(2):188–194
Hsu LJ et al (2000) alpha-synuclein promotes mitochondrial deficit and oxidative stress Am J Pathol, 157(2): pp. 401 – 10
Yang YX, Muqit MM, Latchman DS (2006) Induction of parkin expression in the presence of oxidative stress. Eur J Neurosci 24(5):1366–1372
Shen J, Cookson MR (2004) Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron 43(3):301–304
Liu ZQ et al (2021) Manganese-induced alpha-synuclein overexpression aggravates mitochondrial damage by repressing PINK1/Parkin-mediated mitophagy. Food Chem Toxicol 152:112213
Chaturvedi SK et al (2016) Protein misfolding and aggregation: mechanism. Factors Detect 51(9):1183–1192
Yu J, Lyubchenko YL (2009) Early stages for Parkinson’s development: alpha-synuclein misfolding and aggregation. J Neuroimmune Pharmacol 4(1):10–16
Schlehe JS et al (2008) Aberrant folding of pathogenic parkin mutants: aggregation versus degradation. J Biol Chem 283(20):13771–13779
Zheng Q et al (2016) Dysregulation of Ubiquitin-Proteasome System in neurodegenerative diseases. Front Aging Neurosci 8:303
Peng X et al (2005) Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118(Pt 15):3523–3530
Hyman SE (2005) Neurotransmitters Curr Biol 15(5):R154–R158
Emanuele M, Chieregatti E (2015) Mechanisms of alpha-synuclein action on neurotransmission: cell-autonomous and non-cell autonomous role. Biomolecules 5(2):865–892
Nemani VM et al (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65(1):66–79
Larsen KE et al (2006) Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26(46):11915–11922
Itier JM et al (2003) Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet 12(18):2277–2291
Cremer JN et al (2015) Changes in the expression of neurotransmitter receptors in Parkin and DJ-1 knockout mice–A quantitative multireceptor study. Neuroscience 311:539–551
Forloni G et al (2021) Inflammation and Parkinson’s disease pathogenesis: mechanisms and therapeutic insight. Prog Mol Biol Transl Sci 177:175–202
Li Y et al (2021) Targeting microglial α-synuclein/TLRs/NF-kappaB/NLRP3 inflammasome axis in Parkinson’s disease. Front Immunol 12:719807
Hoenen C et al (2016) Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: stronger effects of the A53T mutant. PLoS ONE 11(9):e0162717
Hoyer W et al (2004) Impact of the acidic C-terminal region comprising amino acids 109 – 140 on α-synuclein aggregation in vitro. Biochemistry 43(51):16233–16242
Hang L, Thundyil J, Lim KL (2015) Mitochondrial dysfunction and Parkinson disease: a Parkin–AMPK alliance in neuroprotection, vol 1350. Annals of the New York Academy of Sciences, pp 37–47. 1
Lipton SA (2007) Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets 8(5):621–632
Hedrich K et al (2004) Distribution, type, and origin of Parkin mutations: review and case studies. Mov Disorders: Official J Mov Disorder Soc 19(10):1146–1157
LeWitt PA (2008) Levodopa for the treatment of Parkinson’s disease. N Engl J Med 359(23):2468–2476
Sliter DA et al (2018) Parkin and PINK1 mitigate STING-induced inflammation. Nature 561(7722):258–262
Sarkar S et al (2017) Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. Npj Parkinson’s Disease 3(1):30
Zhao H et al (2021) Neuroprotective role of akt in Hypoxia Adaptation in Andeans. Front NeuroSci 14:607711
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157(5):1415–1430
Smith WW et al (2005) Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 14(24):3801–3811
Kuroda Y et al (2006) Parkin affects mitochondrial function and apoptosis in neuronal and myogenic cells. Biochem Biophys Res Commun 348(3):787–793
Uzman A (2003) Molecular biology of the cell: Alberts. John Wiley & Sons Inc. USA, Walter, P., K., and
de Oliveira GAP, Silva JL (2019) Alpha-synuclein stepwise aggregation reveals features of an early onset mutation in Parkinson’s disease. Commun Biology 2(1):374
Appel-Cresswell S et al (2013) Alpha‐synuclein p. H50Q, a novel pathogenic mutation for Parkinson’s disease. 28(6):811–813
Guerrero-Ferreira R et al (2019) Two new polymorphic structures of human full-length alpha-synuclein fibrils solved by cryo-electron microscopy. 8:e48907
Pedersen CC et al (2021) A systematic review of associations between common SNCA variants and clinical heterogeneity in Parkinson’s disease. Npj Parkinson’s Disease 7(1):54
Pan F et al (2012) SNP rs356219 of the α-synuclein (SNCA) gene is associated with Parkinson’s disease in a Chinese Han population. Parkinsonism Relat Disord 18:632–634
Sriram SR et al (2005) Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. 14(17):2571–2586
Beasley SA, Hristova VA (2007) S.J.P.o.t.N.A.o.S. Shaw, Structure of the Parkin in-between-ring domain provides insights for E3-ligase dysfunction in autosomal recessive Parkinson’s disease. 104(9):3095–3100
Fiesel FC et al (2015) Structural and functional impact of parkinson disease-associated mutations in the E3 ubiquitin ligase parkin. 36(8):774–786
Terreni L et al (2001) New mutation (R42P) of the parkin gene in the ubiquitinlike domain associated with parkinsonism. Neurology 56(4):463–466
Liu W et al (2009) PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and α-synuclein aggregation. cell Cult Models Parkinson’s Disease 4(2):e4597
Biswas R, Bagchi AJG (2017) A comprehensive computational study on pathogenic mis-sense mutations spanning the RING2 and REP domains of Parkin protein. 610:49–58
Gao T et al (2020) Association of ZNF184, IL1R2, LRRK2, ITPKB, and PARK16 with sporadic Parkinson’s disease in Eastern China. Neurosci Lett 735:135261
Chai C, Lim KL (2013) Genetic insights into sporadic Parkinson’s disease pathogenesis. Curr Genomics 14(8):486–501
Kumaran R, Cookson MR (2015) Pathways to parkinsonism redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum Mol Genet 24(R1):R32–44
Wilkaniec A et al (2019) Extracellular alpha-synuclein oligomers induce parkin S-Nitrosylation: relevance to sporadic Parkinson’s Disease Etiopathology. Mol Neurobiol 56(1):125–140
Choi P et al (2001) Co-association of parkin and alpha-synuclein. NeuroReport 12(13):2839–2843
Khandelwal PJ et al (2010) Parkinson-related parkin reduces α-Synuclein phosphorylation in a gene transfer model. Mol Neurodegener 5:47
Jęśko H, Lenkiewicz AM, Adamczyk A (2017) Treatments and compositions targeting α-synuclein: a patent review (2010–2016). Expert Opin Ther Pat 27(4):427–438
Jęśko H et al (2019) The interplay between parkin and alpha-synuclein; possible implications for the pathogenesis of Parkinson’s disease. Acta Neurobiol Exp (Wars) 79(3):276–289
Singh K et al (2018) Parkin targets NOD2 to regulate astrocyte endoplasmic reticulum stress and inflammation. Glia 66(11):2427–2437
Moszczynska A et al (2007) Parkin disrupts the alpha-synuclein/dopamine transporter interaction: consequences toward dopamine-induced toxicity. J Mol Neurosci 32(3):217–227
Chung E et al (2020) Intracellular delivery of Parkin rescues neurons from accumulation of damaged mitochondria and pathological α-synuclein. Sci Adv 6(18):eaba1193
Paciello O et al (2006) Parkin and its association with alpha-synuclein and AbetaPP in inclusion-body myositis and AbetaPP-overexpressing cultured human muscle fibers. Acta Myol 25(1):13–22
Chung KK et al (2001) Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7(10):1144–1150
Shimura H et al (2001) Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science 293(5528):263–269
Madsen DA et al (2021) Interaction between Parkin and α-synuclein in PARK2-mediated Parkinson’s disease. 10(2):283
Twohig D, Nielsen HMJMN (2019) α-synuclein in the pathophysiology of Alzheimer’s disease. 14(1):1–19
Witte ME et al (2009) Parkinson’s disease-associated parkin colocalizes with Alzheimer’s disease and multiple sclerosis brain lesions. 36(3):445–452
Lücking C, Brice* AJC, CMLS MLS (2000) Alpha-synuclein Parkinson’s Disease 57:1894–1908
Von Coelln R et al (2004) Parkin-associated Parkinson’s disease. 318:p175–184
Lu J-Q et al (2009) Association of α-Synuclein immunoreactivity with inflammatory activity in multiple sclerosis lesions. J Neuropathology Experimental Neurol 68(2):179–189
Wilhelmus MM et al (2012) Involvement and interplay of Parkin, PINK1, and DJ1 in neurodegenerative and neuroinflammatory disorders. 53(4):983–992
Roberts B et al (2022) Synucleinopathy in Amyotrophic Lateral Sclerosis: A Potential Avenue for Antisense Therapeutics? 23(16): p. 9364
Zhang C-W et al (2016) Parkin Regul Neurodegenerative Disorders 7:248
Charles V et al (2000) Alpha-synuclein immunoreactivity of huntingtin polyglutamine aggregates in striatum and cortex of Huntington’s disease patients and transgenic mouse models. 289(1):29–32
Rubio I et al (2009) Effects of partial suppression of parkin on huntingtin mutant R6/1 mice 1281: pp. 91–100
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
K.S.: Writing–original draft. S.C.: Writing – original draft, Conceptualization. A.G.: Writing–original draft, R.S.: Writing – review & editing, Formal analysis and Supervision. R.C.: Writing–original draft. # Authors contributing equally to the manuscript (Kajal Sharma and Shivani Chib).
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, K., Chib, S., Gupta, A. et al. Interplay between α-synuclein and parkin genes: Insights of Parkinson’s disease. Mol Biol Rep 51, 586 (2024). https://doi.org/10.1007/s11033-024-09520-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09520-7