Abstract
Background
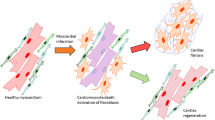
The cardioprotective properties of mesenchymal stem cells and the therapeutic potential of curcumin (CUR) have been explored. Combining these approaches may enhance stem cell effectiveness and expedite healing. This study aimed to investigate the synergistic effects of co-treating bone marrow mesenchymal stem cells (BMSCs) with curcumin on vascular endothelial growth factor (VEGF) levels, in a rat model of myocardial ischemia (MI).
Methods and results
Sixty-five male rats were divided into four groups: G1 (healthy control), G2 (MI induced by isoproterenol hydrochloride), G3 (treated with BMSCs), and G4 (co-treated with curcumin and BMSCs). Blood and tissue samples were collected at specific time points (day 1, 7, 15 and 21) after MI induction. Serum levels of lactate dehydrogenase (LDH), creatine kinase (CK), cardiac troponin I (cTnI), aspartate aminotransferase (AST), CK-MB and VEGF were measured. VEGF mRNA and protein expression were evaluated using RT-qPCR and Western blot techniques. Histopathological assessments were performed using H&E staining and CD31 immunofluorescence staining. VEGF expression significantly increased on days 7 and 15 in the CUR-BMSCs group, peaking on day 7. Western blot analysis confirmed elevated VEGF protein expression on days 7 and 15 post-MI. ELISA results demonstrated increased serum VEGF levels on days 7 and 15, reaching the highest level on day 7 in CUR-BMSCs-treated animals. Treated groups showed lower levels of LDH, AST, CK, CK-MB and cTnI compared to the untreated MI group. H&E staining revealed improved myocardial structure, increased formation of new capillaries, in both treatment groups compared to the MI group.
Conclusion
Combining curcumin with BMSCs promotes angiogenesis in the infarcted myocardium after 15 days of MI induction. These findings suggest the potential of this combined therapy approach for enhancing cardiac healing and recovery.






Similar content being viewed by others
Data availability
Supplementary data and materials associated with this research, only will be available on request and reasonable purpose.
References
Kumar M, Dhatwalia SK, Dhawan D (2016) Role of angiogenic factors of herbal origin in regulation of molecular pathways that control tumor angiogenesis. Tumour Biol 37(11):14341–14354. https://doi.org/10.1007/s13277-016-5330-5
Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, Wang N (2019) VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol 234:17690–17703. https://doi.org/10.1002/jcp.28395
Choi WY, Poss KD (2012) Cardiac regeneration. Curr Top Dev Biol 100:319–344. https://doi.org/10.1016/B978-0-12-387786-4.00010-5
Sun Q, Zhang Z, Sun Z (2014) The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis 1(1):113–119. https://doi.org/10.1016/j.gendis.2014.07.003
Powers N, Huang GN (2022) Visualization of regenerating and repairing hearts. Clin Sci (Lond) 136(10):787–798. https://doi.org/10.1042/CS20211116
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM et al (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010
Pei J, Cai L, Wang F, Xu C, Pei S, Guo H et al (2022) LPA2 contributes to vascular endothelium homeostasis and cardiac remodeling after myocardial infarction. Circ Res 131(5):388–403. https://doi.org/10.1161/CIRCRESAHA
Zhang J, Wang Q, Rao G, Qiu J, He R (2019) Curcumin improves perfusion recovery in experimental peripheral arterial disease by upregulating microRNA-93 expression. Exp Ther Med 17(1):798–802
Rahnavard M, Hassanpour M, Ahmadi M, Heidarzadeh M, Amini H, Javanmard MZ et al (2019) Curcumin ameliorated myocardial infarction by inhibition of cardiotoxicity in the rat model. J Cell Biochem. https://doi.org/10.1002/jcb.28480
Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Kapp RW Jr, Letz G et al (1983) An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals: a report of the US environmental protection agency gene-tox program. Mutat Res 115(1):1–72. https://doi.org/10.1016/0165-1110(83)90014-3
Zhang J, Wang QS, Le TY, Romanazzo S, Rashid FN, Ogawa M, Kilian KA et al (2021) Pluripotent stem cell-derived mesenchymal stromal cells improve cardiac function and vascularity after myocardial infarction. Cytotherapy 23(12):1074–1084. https://doi.org/10.1016/j.jcyt.2021.07.016
Li L, Chen X, Wang WE, Zeng C (2016) How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int 2016:9682757. https://doi.org/10.1155/2016/9682757
Xie N, Li Z, Adesanya TM, Guo W, Liu Y, Fu M et al (2016) Transplantation of placenta-derived mesenchymal stem cells enhances angiogenesis after ischemic limb injury in mice. J Cell Mol Med 20(1):29–37. https://doi.org/10.1111/jcmm.12489
Chehelcheraghi F, Chien S, Bayat M (2019) Mesenchymal stem cells improve survival in ischemic diabetic random skin flap via increased angiogenesis and VEGF expression. J Cell Biochem 120(10):17491–17499. https://doi.org/10.1002/jcb.29013
Mykhaylichenko VY, Kubyshkin A, Samarin S, Fomochkina I, Anisimova L (2016) Experimental induction of reparative morphogenesis and adaptive reserves in the ischemic myocardium using multipotent mesenchymal bone marrow-derived stem cells. Pathophysiology 23(2):95–104. https://doi.org/10.1016/j.pathophys
Beliën H, Evens L, Hendrikx M, Bito V, Bronckaers A (2022) Combining stem cells in myocardial infarction: the road to superior repair? Med Res Rev 42(1):343–373. https://doi.org/10.1002/med.21839
Hua P, Wang YY, Liu LB, Liu JL, Liu JY, Yang YQ et al (2014) In vivo magnetic resonance imaging tracking of transplanted superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells in rats with myocardial infarction. Mol Med Rep 11(1):113–120. https://doi.org/10.3892/mmr.2014.2649
Tork OM, Khaleel EF, Abdelmaqsoud OM (2015) Altered cell to cell communication, autophagy and mitochondrial dysfunction in a model of hepatocellular carcinoma: potential protective effects of curcumin and stem cell therapy. Asian Pac J Cancer Prev 16(18):8271–8279. https://doi.org/10.7314/apjcp.2015.16.18.8271
Ormond DR, Shannon C, Oppenheim J, Zeman R, Das K, Murali R et al (2014) Stem cell therapy and curcumin synergistically enhance recovery from spinal cord injury. PLoS One pretreatment 9(2):e88916. https://doi.org/10.1371/journal.pone.0088916
Kumar M, Kasala ER, Bodduluru LN, Dahiya V, Lahkar M (2016) Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm Res 65(8):613–622. https://doi.org/10.1007/s00011-016-0944-z
Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Waksman R et al (2017) Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res 120(10):1598–1613
Attari F, Zahmatkesh M, Aligholi H, Mehr SE, Sharifzadeh M, Gorji A et al (2015) Curcumin as a double-edged sword for stem cells: dose, time and cell type-specific responses to curcumin. Daru 23:1–7
Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC (2021) Angiogenesis after acute myocardial infarction. Cardiovasc Res 117(5):1257–1273. https://doi.org/10.1093/cvr/cvaa287
Xie Z, Gao M, Batra S, Koyama T (1997) The capillarity of left ventricular tissue of rats subjected to coronary artery occlusion. Cardiovasc Res 33(3):671–676. https://doi.org/10.1016/s0008-6363(96)00250-7
Rodriguez-Porcel M, Cai W, Gheysens O, Willmann JK, Chen K, Wang H et al (2008) Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med 49(4):667–673. https://doi.org/10.2967/jnumed.107.040576
Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons MI (1996) VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol 270(52):H1803–H1811. https://doi.org/10.1152/ajpheart.1996.270.5.H1803
Soeki T, Tamura Y, Shinohara H, Tanaka H, Bando K, Fukuda N (2000) Serial changes in serum VEGF and HGF in patients with acute myocardial infarction. Cardiology 93(3):168–174
Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA et al (2012) Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151(5):1083–1096. https://doi.org/10.1016/j.cell.2012.10.023
Kobayashi K, Maeda K, Takefuji M, Kikuchi R, Morishita Y, Hirashima M et al (2017) Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci Rep 7(1):1–13. https://doi.org/10.1038/s41598-017-07524-x
White SJ, Chong JJ (2020) Mesenchymal stem cells in cardiac repair: effects on myocytes, vasculature, and fibroblasts. Clin Ther 42(10):1880–1891
Bahrami N, Ale-Ebrahim M, Asadi Y, Barikrow N, Salimi A, Roholah F (2023) Combined application of human amniotic membrane mesenchymal stem cells and a modified PGS-co-PCL film in an experimental model of myocardial ischemia-reperfusion injury. Appl Biochem Biotechnol 3:1–8
Yang G, Zhu J, Zhan G, Fan G, Deng L, Tang H et al (2022) Mesenchymal stem cell-derived neuron-like cell transplantation combined with electroacupuncture improves synaptic plasticity in rats with intracerebral hemorrhage via mTOR/p70S6K signaling. Stem Cells Int. 2022:6450527. https://doi.org/10.1155/2022/6450527
Kaur G, Hussain MS, Kataria T, Deb A, Mohapatra C (2021) Health benefits of curcumin in the prevention and treatment of diseases. Int J Pharma Bio Sci 1(7):112–118
Tang J, Xie Q, Pan G, Wang J, Wang M (2006) Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg 30(2):353–361. https://doi.org/10.1016/j.ejcts.2006.02.070
Meissner M, Doll M, Hrgovic I, Reichenbach G, König V, Hailemariam-Jahn T et al (2011) Suppression of VEGFR2 expression in human endothelial cells by dimethylfumarate treatment: evidence for anti-angiogenic action. J Invest Dermatol 131(6):1356–1364. https://doi.org/10.1038/jid.2011.46
Attari F, Zahmatkesh M, Aligholi H, Mehr SE, Sharifzadeh M, Gorji A et al (2015) Curcumin as a double-edged sword for stem cells: dose, time and cell type-specific responses to curcumin. Daru 23(1):33. https://doi.org/10.1186/s40199-015-0115-8
Basak S, Srinivas V, Mallepogu A, Duttaroy AK (2020) Curcumin stimulates angiogenesis through VEGF and expression of HLA-G in first-trimester human placental trophoblasts. Cell Biol Int 44(5):1237–1251. https://doi.org/10.1002/cbin.11324
Lobo Filho HG, Ferreira NL, Sousa RBD, Carvalho ERD, Lobo PLD, Lobo Filho JG (2011) Experimental model of myocardial infarction induced by isoproterenol in rats. Rev Bras Cir Cardiovasc 26:469–476. https://doi.org/10.5935/1678-9741.20110024
Liu J, Zhu P, Song P, Xiong W, Chen H, Peng W et al (2015) Pretreatment of adipose derived stem cells with curcumin facilitates myocardial recovery via antiapoptosis and angiogenesis. Stem Cells Int. https://doi.org/10.1155/2015/638153
Hu C, Liu W, Long L, Wang Z, Zhang W, He S et al (2022) Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomater 290:121849
Chen K, Bai L, Lu J, Chen W, Liu C, Guo E et al (2022) Human decidual mesenchymal stem cells obtained from early pregnancy improve cardiac revascularization postinfarction by activating ornithine metabolism. Front Cardiovasc Med. 9:837780
Liang SX, Phillips WD (2013) Migration of resident cardiac stem cells in myocardial infarction. Anat Rec 296(2):184–191. https://doi.org/10.1002/ar.22633
Casey TM, Arthur PG (2000) Hibernation in noncontracting mammalian cardiomyocytes. Circulation 102(25):3124–3129. https://doi.org/10.1161/01.cir.102.25.3124
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L (2008) Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood J Am Soc Hematol 111(3):1327–1333. https://doi.org/10.1182/blood-2007-02-074997
Yan S, Zhou M, Zheng X, Xing Y, Dong J, Yan M et al (2021) Anti-inflammatory effect of curcumin on the mouse model of myocardial infarction through regulating macrophage polarization. Mediators Inflamm 2021:9976912. https://doi.org/10.1155/2021/9976912
Peluzzo AM, Autieri MV (2022) Challenging the paradigm: anti-inflammatory interleukins and angiogenesis. Cells 11(3):587. https://doi.org/10.3390/cells11030587
Acknowledgements
The authors thank the Student Research Committee, Urmia University of Medical Sciences, Urmia, Iran, for supporting this project.
Funding
The current research was funded by Urmia University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: MZJ HS. Performed the experiments: NM MZJ NA. Analyzed the data: MZJ NA. Wrote the manuscript: NM HS MZJ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethical approval
This study was ethically approved by the Animal Care and Use Committee at Urmia University of Medical Sciences (IR.UMSU.REC.1396.384).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirfakhraie, N., Shoorei, H., Abedpour, N. et al. Co-treatment with bone marrow-derived mesenchymal stem cells and curcumin improved angiogenesis in myocardium in a rat model of MI. Mol Biol Rep 51, 261 (2024). https://doi.org/10.1007/s11033-023-09180-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09180-z