Abstract
Background
Diabetic Mellitus is characterized by a lack or failure of insulin to bind to its target receptor or failure of the pancreas to yield insulin. This study evaluated the antihyperglycemic activity of 14-deoxy, 11, 12-didehydro andrographolide on streptozotocin-nicotinamide-induced type 2 diabetic rats. Diabetic conditions were induced by administering streptozotocin at a dosage of 45 mg/kg body weight and nicotinamide at a dosage of 110 mg/kg body weight through intraperitoneal injection.
Materials and methods
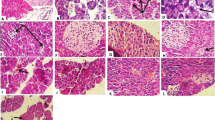
Diabetic-induced rats were treated with 14-deoxy, 11, 12-didehydro andrographolide concentrations between 10 and 500 mg/kg body weight. The blood glucose level and body weight of the rats were periodically examined. The pancreas was isolated and the histopathological staining was performed after making fine sections of the pancreas using a microtome. The influence of 14-deoxy, 11, 12-didehydro andrographolide on the expression level of various insulin signaling cascades was determined with q-PCR and western blotting.
Results
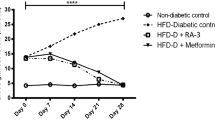
The blood glucose level of the diabetic-induced rats was significantly (p < 0.05) higher when compared with the control group and resulted in a drop in the blood glucose level of the diabetic rats. Oral glucose level was also reduced in the treatment group and no significant reduction was noted in the untreated. The lipid profiling revealed that the atherogenic index and cholesterol ratio was increased in the diabetic group over the control group. Upregulation of the insulin cascades like IRTK and GLUT4 was observed by the q-PCR and upregulation of GLUT4 and IR-β was observed by the western blot analysis.
Conclusion
Overall, the finding indicates that 14-deoxy, 11, 12-didehydro andrographolide exhibited antihyperglycemic activity by modulating the expression of insulin cascades.








Similar content being viewed by others
Data availability
The data used to support the findings of this study areavailable from the corresponding author upon request.
References
Pedersen-Bjergaard U, Thorsteinsson B (2017) Reporting severe hypoglycemia in type 1 diabetes: facts and pitfalls. Curr Diab Rep 17:1–11. https://doi.org/10.1007/S11892-017-0965-1/METRICS
Issac PK, Karan R, Guru A et al (2021) Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Mol Biol Rep 48:5857–5872. https://doi.org/10.1007/S11033-021-06580-X/METRICS
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846. https://doi.org/10.1038/NATURE05482
Bansal P, Paul P, Mudgal J et al (2012) Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol 64:651–658. https://doi.org/10.1016/J.ETP.2010.12.009
Danaei G, Finucane MM, Lu Y et al (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378:31–40. https://doi.org/10.1016/S0140-6736(11)60679-X
Wild S, Roglic G, Green A et al (2004) Global Prevalence of DiabetesEstimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053. https://doi.org/10.2337/DIACARE.27.5.1047
Jimeno C, Sy RA, La PPD et al (2021) Direct medical costs of type 2 diabetes mellitus in the Philippines: findings from two hospital databases and physician surveys. BMJ Open 11:e049737. https://doi.org/10.1136/bmjopen-2021-049737
Akın S, Bölük C (2020) Prevalence of comorbidities in patients with type-2 diabetes mellitus. Prim Care Diabetes 14:431–434. https://doi.org/10.1016/j.pcd.2019.12.006
Barzegar N, Ramezankhani A, Tohidi M et al (2021) Long-term glucose variability and incident cardiovascular diseases and all-cause mortality events in subjects with and without diabetes: Tehran lipid and glucose study. Diabetes Res Clin Pract 178:108942
Nie T, Cooper GJS (2021) Mechanisms Underlying the Antidiabetic Activities of Polyphenolic Compounds: A Review. Front Pharmacol. https://doi.org/10.3389/fphar.2021.798329
Agarwal SM, Panda R, Costa-Dookhan KA et al (2021) Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. https://doi.org/10.1038/s41398-021-01338-2
Abdullahi AA (2011) Trends and challenges of traditional medicine in Africa. Afr J Tradit Complement Altern Med 8:115. https://doi.org/10.4314/AJTCAM.V8I5S.5
Jang A, Kang DH, Kim DU (2017) Complementary and Alternative medicine use and its association with emotional status and quality of life in patients with a solid tumor: a cross-sectional study. J Altern Complement Med 23:362–369. https://doi.org/10.1089/ACM.2016.0289
Salazar-Gómez A, Alonso-Castro AJ (2022) Medicinal plants from latin America with wound healing activity: ethnomedicine, phytochemistry, preclinical and clinical Studies: a review. Pharmaceuticals 15:1095. https://doi.org/10.3390/PH15091095
Chander R, Srivastava V, Tandon And JS, Kapoor NK (2008) Antihepatotoxic activity of diterpenes of andrographis paniculata (Kal-Megh) against plasmodium Berghei-Induced hepatic damage in mastomys natalensis. Int J Pharmacogn 33:135–138. https://doi.org/10.3109/13880209509055213
Xia Y-F, Ye B-Q, Li Y-D et al (2004) Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J Immunol 173:4207–4217. https://doi.org/10.4049/JIMMUNOL.173.6.4207
Wiart C, Kumar K, Yusof MY et al (2005) Antiviral properties of ent-labdene diterpenes of Andrographis paniculata Nees, inhibitors of herpes simplex virus type 1. Phyther Res 19:1069–1070. https://doi.org/10.1002/ptr.1765
Sheeja K, Guruvayoorappan C, Kuttan G (2007) Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int Immunopharmacol 7:211–221. https://doi.org/10.1016/J.INTIMP.2006.10.002
Thakur AK, Soni UK, Rai G et al (2014) Protective effects of andrographis paniculata extract and pure andrographolide against chronic stress-triggered pathologies in rats. Cell Mol Neurobiol 34:1111–1121. https://doi.org/10.1007/s10571-014-0086-1
Anju D, Jugnu G, Kavita S, et al (2012) A review on medicinal prospectives of Andrographis paniculata Nees. J Pharm Sci Innov 1(1):1–4.
Yu BC, Hung CR, Chen WC, Cheng JT (2003) Antihyperglycemic effect of Andrographolide in Streptozotocin-induced diabetic rats. Planta Med 69:1075–1079. https://doi.org/10.1055/s-2003-45185
Pholphana N, Rangkadilok N, Thongnest S et al (2004) Determination and variation of three active diterpenoids in Andrographis paniculata (Burm.f.) Nees. Phytochem Anal 15:365–371. https://doi.org/10.1002/pca.789
Guan S-P, Kong L-R, Cheng C et al (2011) Protective role of 14-deoxy-11,12-didehydroandrographolide, a Noncytotoxic Analogue of Andrographolide, in allergic airway inflammation. J Nat Prod 74:1484–1490. https://doi.org/10.1021/np2002572
Yen C-C, Liu Y-T, Lin Y-J et al (2019) Bioavailability of the diterpenoid 14-deoxy-11,12-didehydroandrographolide in rats and up-regulation of hepatic drug-metabolizing enzyme and drug transporter expression. Phytomedicine 61:152841. https://doi.org/10.1016/j.phymed.2019.152841
Kamaraj N, Rajaguru PY, Issac P, kumar, Sundaresan S, (2017) Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J Pharm Sci 12:353–362. https://doi.org/10.1016/J.AJPS.2017.02.003
Lee S, Morita H, Tezuka Y (2015) Preferentially cytotoxic constituents of andrographis paniculata and their preferential cytotoxicity against human pancreatic cancer cell lines. Nat Prod Commun 10:1153–1158
Tan HK, Muhammad TST, Tan ML (2016) 14-Deoxy-11,12-didehydroandrographolide induces DDIT3-dependent endoplasmic reticulum stress-mediated autophagy in T-47D breast carcinoma cells. Toxicol Appl Pharmacol 300:55–69. https://doi.org/10.1016/j.taap.2016.03.017
Cai W, Li Y, Chen S et al (2015) 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antiviral Res 118:82–92. https://doi.org/10.1016/j.antiviral.2015.03.008
Awang K, Abdullah NH, Hadi AHA, Fong YS (2012) Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J Biomed Biotechnol 2012:876458. https://doi.org/10.1155/2012/876458
Yoopan N, Thisoda P, Rangkadilok N et al (2007) Cardiovascular effects of 14-Deoxy-11,12-didehydroandrographolide and Andrographis paniculata extracts. Planta Med 73:503–511. https://doi.org/10.1055/S-2007-967181/ID/2
Wu TS, Chern HJ, Damu AG et al (2007) Flavonoids and ent-labdane diterpenoids from Andrographis paniculata and their antiplatelet aggregatory and vasorelaxing effects. J Asian Nat Prod Res 10:17–24. https://doi.org/10.1080/10286020701273627
Lee MJ, Rao YK, Chen K et al (2010) Andrographolide and 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata attenuate high glucose-induced fibrosis and apoptosis in murine renal mesangeal cell lines. J Ethnopharmacol 132:497–505. https://doi.org/10.1016/J.JEP.2010.07.057
Szkudelski T (2012) Streptozotocin–nicotinamide-induced diabetes in the rat. Charact Exp Model 237:481–490. https://doi.org/10.1258/EBM.2012.011372
Fröde TS, Medeiros YS (2008) Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol 115:173–183. https://doi.org/10.1016/J.JEP.2007.10.038
Kumar IP, Snega Priya P, Meenatchi R et al (2022) Potential mechanism of Jatropha gossypifolia phenolic derivatives in enhancing insulin-signalling cascades GLUT 4, IRβ and GSK-3β in streptozotocin nicotinamide induced type II diabetic in wistar rat model. J King Saud Univ: Sci 34:102223. https://doi.org/10.1016/J.JKSUS.2022.102223
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502. https://doi.org/10.1093/clinchem/18.6.499
Bhakkiyalakshmi E, Sireesh D, Sakthivadivel M et al (2016) Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via Nrf2 signaling in experimental diabetes. Eur J Pharmacol 777:9–16. https://doi.org/10.1016/J.EJPHAR.2016.02.054
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883/METRICS
Abdollahi M, Zuki ABZ, Goh YM et al (2011) Effects of Momordica charantia on pancreatichistopathological changes associated withstreptozotocin-induced diabetes in neonatal rats. Histol Histopathol 26(1):13–21. https://doi.org/10.14670/HH-26.13.PMID:21117023.
Kumar V, Ahmed D, Gupta PS et al (2013) Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement Altern Med 13:1–19. https://doi.org/10.1186/1472-6882-13-222/FIGURES/25
Arockiaraj J, Vanaraja P, Easwvaran S et al (2011) Gene profiling and characterization of arginine kinase-1 (MrAK-1) from freshwater giant prawn (Macrobrachium rosenbergii). Fish Shellfish Immunol 31:81–89. https://doi.org/10.1016/J.FSI.2011.04.004
Palanisamy R, Kumaresan V, Harikrishnan R et al (2015) Functional roles and gene regulation of tumor necrosis factor receptor 1 in freshwater striped murrel. Mol Immunol 66:240–252. https://doi.org/10.1016/j.molimm.2015.03.015
Liberman Z, Eldar-Finkelman H (2005) Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. J Biol Chem 280:4422–4428. https://doi.org/10.1074/jbc.M410610200
Mohammadi A, Gholamhoseinian A, Fallah H (2014) Zataria multiflora increases insulin sensitivity and PPARγ gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci 17:263
Mozaffarian D, Maki KC, Bays HE et al (2022) Effectiveness of a Novel $ω$-3 Krill Oil Agent in Patients With Severe Hypertriglyceridemia. JAMA Netw Open 5:e2148191
Kanoni S, Graham SE, Wang Y et al (2021) Implicating genes, pleiotropy, and sexual dimorphism at blood lipid loci through multi-ancestry meta-analysis. Genome Biol 23(4):2872
Si S, Hou L, Chen X et al (2021) Exploring the causal roles of circulating remnant lipid profile on cardiovascular and cerebrovascular diseases: mendelian randomization study. J Epidemiol 32:205–214
Wadström BN, Wulff AB, Pedersen KM et al (2021) Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study. Eur Heart J 43(34):3258–3269
Nguyen T, Pham T, Nguyen TV (2023) Cardiovascular disease in older patients with end-stage renal disease and chronic dialysis in Vietnam. Eur Heart J 44(1):150. https://doi.org/10.1093/eurheartj/ehac779.116
Shah PP, Brady TM, Meyers KE et al (2021) Association of obesity with cardiovascular risk factors and kidney disease outcomes in primary proteinuric glomerulopathies. Nephron 145:245–255
Kant R, Naithani M, Jain G et al (2021) Quantifying insulin resistance (HOMA-IR) to predict mortality in multiorgan dysfunction syndrome. Indian J Crit Care Med. 25(12):1364–1369
Weng Y, Yu L, Cui J et al (2014) Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J Ethnopharmacol 152:553–560. https://doi.org/10.1016/J.JEP.2014.02.001
Mizukami H, Kudoh K (2021) Diversity of pathophysiology in type 2 diabetes shown by islet pathology. J Diabetes Investig 13:13–16
Charles MA, Leslie RD (2021) Diabetes: concepts of $β$-cell organ dysfunction and failure would lead to earlier diagnoses and prevention. Diabetes 70:2444–2456
Zhang J, Bai J, Zhou Q et al (2022) Glutathione prevents high glucose-induced pancreatic fibrosis by suppressing pancreatic stellate cell activation via the ROS/TGFβ/SMAD pathway. Cell Death Dis 13:440. https://doi.org/10.1038/s41419-022-04894-7
Fu W, Li H, Li T et al (2021) Pentadecanoic acid promotes basal and insulin-stimulated glucose uptake in C2C12 myotubes. Food & Nutr Res. https://doi.org/10.29219/fnr.v65.4527
Kwon E-B, Kang M-J, Ryu HW et al (2020) Acacetin enhances glucose uptake through insulin-independent GLUT4 translocation in L6 myotubes. Phytomedicine 68:153178. https://doi.org/10.1016/j.phymed.2020.153178
Guru A, Issac PK, Saraswathi NT et al (2021) Deteriorating insulin resistance due to WL15 peptide from cysteine and glycine-rich protein 2 in high glucose-induced rat skeletal muscle L6 cells. Cell Biol Int 45:1698–1709. https://doi.org/10.1002/CBIN.11608
Im SS, Kwon SK, Kang SY et al (2006) Regulation of GLUT4 gene expression by SREBP-1c in adipocytes. Biochem J 399:131–139. https://doi.org/10.1042/BJ20060696
Funding
This study didn’t receive any specific funding from public, corporate, or not-for-profit funding entities.
Author information
Authors and Affiliations
Contributions
NK, PKI, KV: study design, manuscript preparation, result analysis.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The Institutional Animal Ethics Committee (53/IAEC/2011) examined and sanctioned the research study.
Consent to participate
All the authors listed in the manuscript have approved the manuscript.
Consent for publication
The data provided in the manuscript isapproved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamaraj, N., Velumani, K., Guru, A. et al. Antihyperglycemic activity of 14-deoxy, 11, 12-didehydro andrographolide on streptozotocin-nicotinamide induced type 2 diabetic rats. Mol Biol Rep 50, 9875–9886 (2023). https://doi.org/10.1007/s11033-023-08878-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08878-4