Abstract
Background
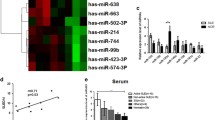
Gene regulation by microRNA (miRNA) is central in T lymphocytes differentiation processes. Here, we investigate miRNA-29b (miR-29b) roles in the reprogramming of T cell differentiation, which can be a promising therapeutic avenue for various types of inflammatory disorders such as rheumatoid arthritis and multiple sclerosis.
Methods and results
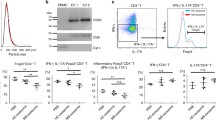
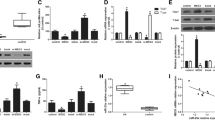
Adipose Mesenchymal Stem Cell-derived exosomes (AMSC-Exo) enriched with miR-29b were delivered into naive CD4+ T (nCD4+) cells. The expression level of important transcription factors including RAR-related orphan receptor gamma (RORγt), GATA3 binding protein (GATA3), T-box transcription factor 21, and Forkhead box P3 was determined by quantitative Real-Time PCR. Moreover, flow cytometry and Enzyme-linked Immunosorbent Assay were respectively used to measure the frequency of T regulatory cells and the levels of cytokines production (Interleukin 17, Interleukin 4, Interferon-gamma, and transforming growth factor beta. This study indicates that the transfection of miR-29b mimics into T lymphocytes through AMSC-Exo can alter the CD4+ T cells’ differentiation into other types of T cells.
Conclusions
In conclusion, AMSC-Exo-based delivery of miR-29b can be considered as a new fascinating avenue for T cell differentiation inhibition and the future treatment of several inflammatory disorders.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors, [Hossein Ghanbarian and Seyed Mahmoud Hashemi], upon reasonable request at [hghanbarian@sbmu.ac.ir, Ghanbarian.hossein@gmail.com and smmhashemi@sbmu.ac.ir].
References
Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R et al (2013) Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498(7452):113–117
Zhou L, Chong MMW, Littman DR (2009) Plasticity of CD4+ T cell lineage differentiation. Immunity 30(5):646–655
Rudd BD (2020) Neonatal T, cells: a reinterpretation. Annu Rev Immunol 38:229–247
O’Shea JJ, Paul WE (2010) Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327(5969):1098–1102
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100(6):655–669
Groom JR (2019) Regulators of T-cell fate: integration of cell migration, differentiation and function. Immunol Rev 289(1):101–114
Coomes SM, Pelly VS, Wilson MS (2013) Plasticity within the alphabeta(+)CD4(+) T-cell lineage: when, how and what for? Open Biol 3(1):120157
Mehta A, Baltimore D (2016) MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol 16(5):279–294
Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CDC et al (2011) MicroRNA-29 regulates T-Box transcription factors and Interferon-g; production in helper T cells. Immunity 35(2):169–181
Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M et al (2011) The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol 12(9):861–869
Fu Y, Chen J, Huang Z (2019) Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA 1(1):24
Dasgupta I, Chatterjee A (2021) Recent advances in miRNA Delivery Systems. Methods Protoc 4(1):10
Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H et al (2019) Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem 120(11):18854–18861
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6(4):287–296
Simons M, Raposo G (2009) Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21 4:575–581
Ji C, Guo X (2019) The clinical potential of circulating microRNAs in obesity. Nat Rev Endocrinol 15(12):731–743
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
Bolandi Z, Hosseini Rad SMA, Soudi S, Hashemi SM, Ghanbarian H (2018) A simple and highly efficient method for transduction of human adipose-derived mesenchymal stem cells. J Cell Biochem. https://doi.org/10.1002/jcb.27453
Bolandi Z, Mokhberian N, Eftekhary M, Sharifi K, Soudi S, Hashemi SM et al (2020) Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4+ T cell. Life Sci. https://doi.org/10.1016/j.lfs.2020.118218
Smith KM, Guerau-de-Arellano M, Costinean S, Williams JL, Bottoni A, Mavrikis Cox G et al (2012) miR-29ab1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. J Immunol 189(4):1567–1576
Kwon JJ, Factora TD, Dey S, Kota J (2018) A systematic review of miR-29 in cancer. Mol Ther Oncolytics 12:173–194
Xu H, Cheung IY, Guo HF, Cheung NKV (2009) MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res 69(15):6275–6281
Szabo SJ, Sullivan BM, Peng SL, Glimcher LH (2003) Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 21(1):713–758
Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z et al (2016) Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res 64(4):831–840
Hosseini K, Ranjbar M, Pirpour Tazehkand A, Asgharian P, Montazersaheb S, Tarhriz V et al (2022) Evaluation of exosomal non-coding RNAs in cancer using high-throughput sequencing. J Transl Med 20(1):30
Luckheeram RV, Zhou R, Verma AD, Xia B (2012) CD4+ T cells: differentiation and functions. Clin Dev Immunol 2012:925135
Gorelik L, Constant S, Flavell RA (2002) Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 195(11):1499–1505
Chauhan SK, Saban DR, Lee HK, Dana R (2009) Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol 182(1):148–153
Xuan J, Guo S, lin, Huang A, Xu H, bing, Shao M, Yang Y et al (2017) MiR-29a and miR-652 attenuate liver fibrosis by inhibiting the differentiation of CD4+ T cells. Cell Struct Funct 42(2):95–103
Brain O, Owens BMJ, Pichulik T, Allan P, Khatamzas E, Leslie A et al (2013) The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity 39(3):521–536
Acknowledgements
We would like to thank the Iranian Stem Cell Council and Shahid Beheshti University of Medical Sciences for their valuable support.
Funding
This study was supported by the Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 18593).
Author information
Authors and Affiliations
Contributions
ZB: Investigation, Formal analysis, Methodology, Data curation, Writing—original draft, Writing—review & editing, SMH: Supervision, Project administration, Conceptualization, Writing—review & editing, MA: Investigation, Writing—original draft, SA: Investigation, Writing—original draft, NM: Writing—review & editing, MM: Investigation, HG: Conceptualization, Funding acquisition, Visualization, Project administration, Supervision, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interest
The authors have no financial interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bolandi, Z., Hashemi, S.M., Abasi, M. et al. In vitro naive CD4+ T cell differentiation upon treatment with miR-29b-loaded exosomes from mesenchymal stem cells. Mol Biol Rep 50, 9037–9046 (2023). https://doi.org/10.1007/s11033-023-08767-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08767-w