Abstract
Background
Accumulating evidence suggests that polo-like kinase 3 (PLK3) plays an essential role in tumor cells and induces cell proliferation and may have implications for the prognosis of various cancers. We sought to define the role of PLK3-dependent proneural–mesenchymal transition (PMT) in the glioblastoma (GBM) therapy.
Methods and results
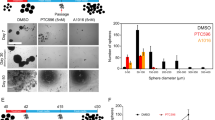
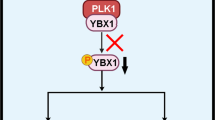
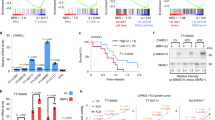
We analyzed the expression data for PLK3 by using the TCGA database. PLK3 expression in GBM cell lines was determined by qRT-PCR and Western blotting. PLK3 levels were modulated using Lentivirus infection, and the effects on symptoms, tumor volume, and survival in mice intracranial xenograft models were determined. Irradiation (IR) was performed to induce PMT. PLK3 expression was significantly elevated in mesenchymal subtype GBM and promoted tumor proliferation in GBM. Additionally enriched PLK3 expression could be associated with poor prognosis in GBM patients compared with those who have lower PLK3 expression. Mechanically, PLK3-dependent PMT induced radioresistance in GBM cells via transcriptional regulation of complement C5a receptor 1 (C5AR1). In therapeutic experiments conducted in vitro, targeting PLK3 by using small molecule inhibitor decreased tumor growth and radioresistance of GBM cells both in vitro and in vivo.
Conclusions
PLK3-C5AR1 axis induced PMT thus enhanced radioresistance in GBM and could become a novel potential therapeutic target for GBM.





Similar content being viewed by others
References
Ostrom QT et al (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. https://doi.org/10.1093/neuonc/noz150
Lee Y et al (2015) FoxM1 promotes stemness and radio-resistance of glioblastoma by regulating the master stem cell regulator Sox2. PLoS ONE. https://doi.org/10.1371/journal.pone.0137703
Wang L-B et al (2021) Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell. https://doi.org/10.1016/j.ccell.2021.01.006
Verhaak RG et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. https://doi.org/10.1016/j.ccr.2009.12.020
Barreau O et al (2013) Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2012-2993
Noushmehr H et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. https://doi.org/10.1016/j.ccr.2010.03.017
Bhat KPL et al (2013) Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. https://doi.org/10.1016/j.ccr.2013.08.001
Li Q, Xie W, Wang N, Li C, Wang M (2018) CDC7-dependent transcriptional regulation of RAD54L is essential for tumorigenicity and radio-resistance of glioblastoma. Transl Oncol. https://doi.org/10.1016/j.tranon.2018.01.003
Nakano I (2014) Proneural-mesenchymal transformation of glioma stem cells: do therapies cause evolution of target in glioblastoma? Future oncology. https://doi.org/10.2217/fon.14.86
Fedele M, Cerchia L, Pegoraro S, Sgarra R, Manfioletti G (2019) Proneural-mesenchymal transition: phenotypic plasticity to acquire multitherapy resistance in glioblastoma. Int J Mol Sci. https://doi.org/10.3390/ijms20112746
Kim SH et al (2016) Serine/Threonine kinase MLK4 determines mesenchymal identity in glioma stem cells in an NF-kappaB-dependent manner. Cancer Cell. https://doi.org/10.1016/j.ccell.2016.01.005
Cheng P et al (2016) FOXD1-ALDH1A3 signaling is a determinant for the self-renewal and tumorigenicity of mesenchymal glioma stem cells. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-15-2860
Cheng P et al (2015) Kinome-wide shRNA screen identifies the receptor tyrosine kinase AXL as a key regulator for mesenchymal glioblastoma stem-like cells. Stem cell reports. https://doi.org/10.1016/j.stemcr.2015.03.005
Wang J et al (2016) Cyclin-dependent kinase 2 promotes tumor proliferation and induces radio resistance in glioblastoma. Translational Oncol. https://doi.org/10.1016/j.tranon.2016.08.007
Jones NM, Rowe MR, Shepherd PR, McConnell MJ (2016) Targeted inhibition of dominant PI3-kinase catalytic isoforms increase expression of stem cell genes in glioblastoma cancer stem cell models. Int J Oncol. https://doi.org/10.3892/ijo.2016.3510
Park SY, Piao Y, Thomas C, Fuller GN, de Groot JF (2016) Cdc2-like kinase 2 is a key regulator of the cell cycle via FOXO3a/p27 in glioblastoma. Oncotarget. https://doi.org/10.18632/oncotarget.8471
Wang J et al (2017) Targeting NEK2 attenuates glioblastoma growth and radioresistance by destabilizing histone methyltransferase EZH2. J Clin Investig. https://doi.org/10.1172/JCI89092
Jia Wang YX, Bai X, Wang N, Yu H, Deng Z, Lian M, Yu S, Liu H, Wanfu Xie, Maode Wang (2018) Targeting dual specificity protein kinase TTK attenuates tumorigenesis of glioblastoma. Oncotarget 9:3081–3088
Raab CA, Raab M, Becker S, Strebhardt K (2021) Non-mitotic functions of polo-like kinases in cancer cells. Biochim Biophys Acta Rev Cancer. https://doi.org/10.1016/j.bbcan.2020.188467
Ding Y, Liu H, Zhang C, Bao Z, Yu S (2022) Polo-like kinases as potential targets and PLK2 as a novel biomarker for the prognosis of human glioblastoma. Aging. https://doi.org/10.18632/aging.203940
Xie M et al (2015) Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. https://doi.org/10.18632/oncotarget.5599
Helmke C, Becker S, Strebhardt K (2016) The role of Plk3 in oncogenesis. Oncogene. https://doi.org/10.1038/onc.2015.105
Vaughan CA et al (2021) The oncogenicity of tumor-derived mutant p53 is enhanced by the recruitment of PLK3. Nat Commun. https://doi.org/10.1038/s41467-021-20928-8
Iseri K et al (2016) Therapeutic effects and mechanism of conditioned media from human mesenchymal stem cells on anti-GBM glomerulonephritis in WKY rats. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00165.2016
Mao P et al (2013) Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1221478110
Halliday J et al (2014) In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1321014111
Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ (2000) Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res 60:6826–6831
Bahassi el M et al (2002) Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. https://doi.org/10.1038/sj.onc.1205850
Bahassi el M, Myer DL, McKenney RJ, Hennigan RF, Stambrook PJ (2006) Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutat Res. https://doi.org/10.1016/j.mrfmmm.2005.12.002
Hao W et al (2016) MicroRNA-206 inhibited the progression of Glioblastoma through BCL-2. J Mol neuroscience: MN. https://doi.org/10.1007/s12031-016-0824-6
Wiese AV et al (2017) The C5a/C5aR1 axis controls the development of experimental allergic asthma independent of LysM-expressing pulmonary immune cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0184956
Ou B et al (2021) C5aR1-positive neutrophils promote breast cancer glycolysis through WTAP-dependent m6A methylation of ENO1. Cell Death Dis. https://doi.org/10.1038/s41419-021-04028-5
Natarajan N et al (2018) Complement receptor C5aR1 plays an evolutionarily conserved role in successful cardiac regeneration. Circulation https://doi.org/10.1161/CIRCULATIONAHA.117.030801
Atanes P et al (2018) C3aR and C5aR1 act as key regulators of human and mouse beta-cell function. Cell Mol Life Sci. https://doi.org/10.1007/s00018-017-2655-1
Li K et al (2017) C5aR1 promotes acute pyelonephritis induced by uropathogenic E. coli. JCI insight 2 https://doi.org/10.1172/jci.insight.97626
Bergdolt S et al (2017) Osteoblast-specific overexpression of complement receptor C5aR1 impairs fracture healing. PLoS ONE. https://doi.org/10.1371/journal.pone.0179512
Xu D et al (2023) C5aR1 promotes the progression of colorectal cancer by EMT and activating Wnt/β-catenin pathway. Clin Transl Oncol. https://doi.org/10.1007/s12094-022-02956-y
Kong F et al (2021) Hepatitis B Virus core protein mediates the upregulation of C5α receptor 1 via NF-κB pathway to facilitate the growth and migration of hepatoma cells. Cancer Res Treat. https://doi.org/10.4143/crt.2020.397
Acknowledgements
This study was supported by the Exploration and Innovation Project of First Affiliated Hospital of Xi'an Jiaotong University (No. 2022MS-15); Shaanxi Provincial Key Research and Development Program (No. 2021KW-51); Natural Science Basic Research Plan in Shaanxi Province of China (No. 2022JQ-791); and Special Fund for Personnel Training of Second Affiliated Hospital of Xi'an Jiaotong University (No. RC(XM)202007).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by LL, YL, QN, and TH. The first draft of the manuscript was written by SY and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the declaration of Helsinki, and all experimental procedures followed the guidelines of the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research. Approval was granted by the Ethics Committee of the first affiliated hospital of Xi'an Jiaotong University (Approval Number: 2021-695).
Consent statement
All patients included in the study provided informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, S., Lv, L., Li, Y. et al. PLK3 promotes the proneural–mesenchymal transition in glioblastoma via transcriptional regulation of C5AR1. Mol Biol Rep 50, 8249–8258 (2023). https://doi.org/10.1007/s11033-023-08716-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08716-7