Abstract
Background
Colorectal Cancer (CC) is among the most prevalent cancers in elderly persons. Radiotherapy is usually prescribed as CC develops, however, radiation beams indiscriminately affect normal cells. Previous studies nominated that probiotics and their metabolites can be used to minimize the side effects of radiotherapy. Hereby, the aim of this study was to investigate the probable correlation between cell-free supernatant of Bacillus subtilis and radiation response in normal and cancerous cell lines.
Methods and results
IEC-18 and SW-48 cells were treated with different concentrations of B. subtilis supernatant. To evaluate the effect of probiotic treatments under radiation and the normal situation, the cytotoxicity of the treatments was measured using the MTT method. The cell cycle status was analyzed by flow cytometry. The expression levels of Bax, Bcl-2, and Caspase 3 genes were also determined by real-time (RT) PCR. B. subtilis supernatant increased the viability of normal cells under radiation treatment, although this effect was not significant. 40% v/v of this mixture could amplify the lethal effect of radiation and decreased the viability of cancer cells. SW-48 cells that received 40% v/v of the supernatant had a significantly higher rate of apoptosis. Probiotic supernatant effectively induced the expression of proapoptotic Bax and Caspase 3 genes.
Conclusion
Presented results confirmed that the supernatant of B. subtilis can be supposed as a clue to improve the efficacy of radiation therapy in CC patients as it increased the sensitivity of cancerous cells and protected normal epithelial cells from detrimental effects of radiation.
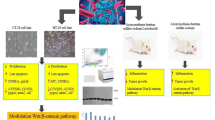
Graphical abstract





Similar content being viewed by others
Data availability
Not applicable.
References
Argilés JM, Busquets S, Stemmler B, López-Soriano FJ (2014) Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14:754–762
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. Ca Cancer J Clin 73:17–48
Rayan A, Raiyn J, Falah M (2017) Nature is the best source of anticancer drugs: indexing natural products for their anticancer bioactivity. PLoS ONE 12:e0187925
Haque MU, Ferdiousi N, Sajon SR (2016) Anticancer agents derived from plant and dietary sources: a review. Int J Pharmacogn 32:55–66
Shah U, Shah R, Acharya S, Acharya N (2013) Novel anticancer agents from plant sources. Chin J Nat Med 11:16–23
Zhang Y, Liu C, Wu C, Song L (2023) Natural peptides for immunological regulation in cancer therapy: mechanism, facts and perspectives. Biomed Pharmacother 159:114257
Ashrafizadeh M, Zarrabi A, Karimi-Maleh H et al (2023) (Nano) platforms in bladder cancer therapy: challenges and opportunities. Bioeng Transl Med 8:e10353
Altun I, Sonkaya A (2018) The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran J Public Health 47:1218–1219
Majeed H, Gupta V (2022) Adverse effects of radiation therapy; in StatPearls [Internet]. StatPearls Publishing, Florida
Artym J, Zimecki M (2023) Colostrum proteins in protection against therapy-induced injuries in cancer chemo-and radiotherapy: a comprehensive review. Biomedicines 11:114
Diffenderfer ES, Verginadis II, Kim MM et al (2020) Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiation Oncology “Biology” Phys 106:440–448
Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97:809–817
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63:3615–3626
Poormontaseri M, Hosseinzadeh S, Shekarforoush SS, Kalantari T (2017) The effects of probiotic Bacillus subtilis on the cytotoxicity of clostridium perfringens type a in Caco-2 cell culture. BMC Microbiol 17:1–8
Sudan S, Zhan X, Li J (2022) A novel probiotic Bacillus subtilis strain confers cytoprotection to host pig intestinal epithelial cells during enterotoxic Escherichia coli infection. Microbiol Spectr 10:e01257–e01221
Khalighi A, Behdani R, Kouhestani S (2016) Probiotics: a comprehensive review of their classification, mode of action and role in human nutrition. Probiotic Prebiotics Human Nutr Health 10:63646
Sil M, Mitra S, Goswami A (2023) Probiotics and immunity: An overview. Viral, Parasitic, Bacterial, and Fungal Infections, Academic Press, Cambridge, p847–861.
Rezaee P, Kermanshahi RK, Falsafi T (2019) Antibacterial activity of lactobacilli probiotics on clinical strains of helicobacter pylori. Iran J Basic Med Sci 22:1118
Weiss G, Christensen HR, Zeuthen LH, Vogensen FK, Jakobsen M, Frøkiær H (2011) Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine 56:520–530
Fusco A, Savio V, Cimini D et al (2023) In vitro evaluation of the most active probiotic strains able to improve the intestinal barrier functions and to prevent inflammatory diseases of the gastrointestinal system. Biomedicines 11:865
Dahiya D, Nigam PS (2023) Biotherapy using probiotics as therapeutic agents to restore the gut microbiota to relieve gastrointestinal tract inflammation, IBD, IBS and prevent induction of cancer. Int J Mol Sci 24:5748
Cadet J, Angelov D, Wagner JR (2022) Hydroxyl radical is predominantly involved in oxidatively generated base damage to cellular DNA exposed to ionizing radiation. Int J Radiat Biol 98:1684–1690
Singh KV, Prakash C, Nirala JP, Nanda RK, Rajamani P (2023) Acute radiofrequency electromagnetic radiation exposure impairs neurogenesis and causes neuronal DNA damage in the young rat brain. Neurotoxicology 94:46–58
Espinal A, Epperly MW, Mukherjee A et al (2022) Intestinal radiation protection and mitigation by second-generation probiotic lactobacillus-reuteri engineered to deliver Interleukin-22. Int J Mol Sci 23:5616
Ciorba MA, Riehl T, Hyun Y-J, Stenson W (2008) M1198 Lactobacillus Rhamnosus GG prevents radiation induced-small intestinal injury in a MyD88 independent, but COX2 dependent manner. Gastroenterology 4:A-359
Binda S, Hill C, Johansen E et al (2020) Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front Microbiol 11:1662
Kaur S, Kaur R, Rani N, Sharma S, Joshi M (2021) Sources and selection criteria of probiotics. Adv Probiotics Sustain Food Med 21:27–43
Chen HH, Kuo MT (2017) Improving radiotherapy in cancer treatment: promises and challenges. Oncotarget 8:62742
Hubenak JR, Zhang Q, Branch CD, Kronowitz SJ (2014) Mechanisms of injury to normal tissue after radiotherapy: a review. Plast Reconstr Surg 133:49e
Häfner MF, Debus J (2016) Radiotherapy for colorectal cancer: current standards and future perspectives. Visc Med 32:172–177
Zhang S, Wang Q, Zhou C et al (2019) Colorectal cancer, radiotherapy and gut microbiota. Chin J Cancer Res 31:212
Venkidesh BS, Shankar SR, Narasimhamurthy RK, Rao SBS, Mumbrekar KD (2023) Radioprotective potential of probiotics against gastrointestinal and neuronal toxicity: a preclinical study. Clin Transl Oncol. https://doi.org/10.1007/s12094-023-03184-8
Changizi V, Azadbakht O, Ghanavati R, Behrouj H, Motevaseli E, Khanzadeh P (2021) Effect of Lactobacillus species on apoptosis-related genes BCL2, BAX, and caspase 3 in the testes of gamma-irradiated rats. Revista da Associação Médica Brasileira 67:1581–1585
Sharma S, Singh R, Kakkar P (2011) Modulation of Bax/Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem Toxicol 49:770–779
Karimi Ardestani S, Tafvizi F, Tajabadi Ebrahimi M (2019) Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum Exp Toxicol 38:1069–1081
Kim S-y, Kim JY, Kim S-H et al (2007) Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Lett 581:865–871
Taherian-Esfahani Z, Abedin-Do A, Nouri Z, Mirfakhraie R, Ghafouri-Fard S, Motevaseli E (2016) Lactobacilli differentially modulate mTOR and Wnt/β-catenin pathways in different cancer cell lines. Iran J Cancer Prev 9(3):e5369
Escamilla J, Lane MA, Maitin V (2012) Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer 64:871–878
Shao Y, Wang X-y, Qiu X, Niu L-l, Ma Z-l (2021) Isolation and purification of a new Bacillus Subtilis strain from deer dung with anti-microbial and anti-cancer activities. Curr Med Sci 41:832–840
Vo TTT, Liu J-F, Wu C-Z, Lin W-N, Chen Y-L, Lee I-T (2020) Surfactin from Bacillus subtilis induces apoptosis in human oral squamous cell carcinoma through ROS-regulated mitochondrial pathway. J Cancer 11:7253
Khosrovan Z, Haghighat S, Mahdavi M (2020) The probiotic bacteria induce apoptosis in breast and colon cancer cells: an immunostimulatory effect. Immunoregulation 3:37–50
Sirpu Natesh N, Arumugam M, Karanam G (2018) Apoptotic role of marine sponge symbiont Bacillus subtilis NMK17 through the activation of caspase-3 in human breast cancer cell line. Mol Biol Rep 45:2641–2651
Aimaier R, Li H, Cao W et al (2022) The secondary metabolites of Bacillus subtilis strain Z15 induce apoptosis in hepatocellular carcinoma cells. Res Square. https://doi.org/10.21203/rs.3.rs-2330612/v1
Dan AK, Manna A, Ghosh S et al (2021) Molecular mechanisms of the lipopeptides from Bacillus subtilis in the apoptosis of cancer cells-a review on its current status in different cancer cell lines. Adv Cancer Biol-Metastasis 3:100019
Liu J, Liu C, Yue J (2021) Radiotherapy and the gut microbiome: facts and fiction. Radiat Oncol 16:1–15
Lu K, Dong S, Wu X, Jin R, Chen H (2021) Probiotics in cancer. Front Oncol 11:638148
Qiu G, Yu Y, Wang Y, Wang X (2019) The significance of probiotics in preventing radiotherapy-induced diarrhea in patients with cervical cancer: a systematic review and meta-analysis. Int J Surg 65:61–69
Acknowledgements
Not applicable.
Funding
This study was funded by Tehran University of Medical Sciences (TUMS).
Author information
Authors and Affiliations
Contributions
NN: Investigation; FZ: Investigation, Writing—Review and Editing, Visualization; MA: Writing—Original Draft, MHG: Supervision, Conceptualization; EM: Supervision, Project Administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no conflict of interest.
Ethical approval
The presented study complies with all ethical considerations under the supervision of Tehran university of medical science (TUMS) ethical committee.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazari, N., Zandsalimi, F., Abdolhosseini, M. et al. Bacillus subtilis supernatant improves the efficacy of radiation therapy in rat intestinal epithelial cells by upregulation of bax and caspase-3 genes. Mol Biol Rep 50, 7639–7647 (2023). https://doi.org/10.1007/s11033-023-08694-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08694-w