Abstract
Background
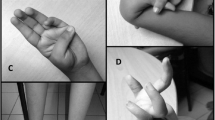
Mutations within the COL12A1 gene have been linked with the onset of congenital Ullrich muscular dystrophy 2 (UCMD2) and Bethlem myopathy. The severity of the symptoms exhibited is dependent on the mutation’s type and whether it is heterozygous or homozygous.
Methods
We used whole-exome sequencing to identify disease-causing variants in a nine-year-old Iranian patient who had weakness, joint contractures, delayed motor development, and other symptoms. We confirmed the pathogenicity of the identified variant using in silico tools and verified its novelty using various databases. We also performed a co-segregation study and confirmed the presence of the variant in the patient’s parents by Sanger sequencing.
Results
Our analysis identified a novel homozygous missense variant in the affected patient in COL12A1 (c.8828 C > T; p.Pro2943Leu). This is the second reported family with UCMD2 caused by a mutation in COL12A1. Our findings confirm that this mutation results in significantly more severe symptoms than Bethlem myopathy.
Conclusion
Our investigation contributes to the expanding body of evidence that links mutations in COL12A1 with UCMD2. Our findings confirm that the homozygous mutation in COL12A1 caused this condition and suggest that genetic testing for this mutation may be useful for diagnosing patients with this disease.






Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Chiquet M, Birk DE, Bönnemann CG, Koch M, Collagen XII (2014) Protecting bone and muscle integrity by organizing collagen fibrils. Int J Biochem Cell Biol 53:51–54
Snellman A, Keränen M-R, Hägg PO, Lamberg A, Hiltunen JK, Kivirikko KI et al (2000) Type XIII collagen forms homotrimers with three triple helical collagenous domains and its association into disulfide-bonded trimers is enhanced by prolyl 4-hydroxylase. J Biol Chem 275(12):8936–8944
Keesler DA, Slobodianuk TL, Kochelek CE, Skaer CW, Haberichter SL, Flood VH (2021) Fibronectin binding to von Willebrand factor occurs via the A1 domain. Res Pract Thromb Haemostasis 5(5):e12534
Bardakov SN, Deev RV, Magomedova RM, Umakhanova ZR, Allamand V, Gartioux C et al (2021) Intrafamilial phenotypic variability of collagen VI-related myopathy due to a new mutation in the COL6A1 gene. J Neuromuscul Dis 8(2):273–285
Kutluk MG, Kadem N, Bektas O, Randa NC, Tuncer GO, Albayrak P et al (2021) A novel variant of COL6A2 gene causing bethlem myopathy and evaluation of essential hypertension. Ann Indian Acad Neurol 24(2):280
Marakhonov AV, Tabakov VY, Zernov NV, Dadali EL, Sharkova IV, Skoblov MY (2018) Two novel COL6A3 mutations disrupt extracellular matrix formation and lead to myopathy from Ullrich congenital muscular dystrophy and Bethlem myopathy spectrum. Gene 672:165–171
Punetha J, Kesari A, Hoffman EP, Gos M, Kamińska A, Kostera-Pruszczyk A et al (2017) Novel Col12A1 variant expands the clinical picture of congenital myopathies with extracellular matrix defects. Muscle Nerve 55(2):277–281
Baker NL, Mörgelin M, Peat R, Goemans N, North KN, Bateman JF et al (2005) Dominant collagen VI mutations are a common cause of Ullrich congenital muscular dystrophy. Hum Mol Genet 14(2):279–293
Zou Y, Zwolanek D, Izu Y, Gandhy S, Schreiber G, Brockmann K et al (2014) Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum Mol Genet 23(9):2339–2352
Bönnemann CG (2011) The collagen VI-related myopathies: Ullrich congenital muscular dystrophy and Bethlem myopathy. Handb Clin Neurol 101:81–96
Cassandrini D, Trovato R, Rubegni A, Lenzi S, Fiorillo C, Baldacci J et al (2017) Congenital myopathies: clinical phenotypes and new diagnostic tools. Ital J Pediatr 43:1–16
Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31(13):3812–3814
Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M (2019) CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 47(D1):D886–D94
Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11(4):361–362
Adzhubei I, Jordan DM, Sunyaev SR (2013) Predicting functional effect of human missense mutations using PolyPhen-2. Curr protocols Hum Genet 76(1):7 1–7. 41
Thomas PD, Ebert D, Muruganujan A, Mushayahama T, Albou LP, Mi H (2022) PANTHER: making genome-scale phylogenetics accessible to all. Protein Sci 31(1):8–22
Capriotti E, Fariselli P, Casadio R (2005) I-Mutant2. 0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res 33(suppl2):W306–W10
Cheng J, Randall A, Baldi P (2006) Prediction of protein stability changes for single-site mutations using support vector machines. Proteins Struct Funct Bioinform 62(4):1125–1132
Mering Cv, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 31(1):258–261
Sievers F, Higgins DG (2014) Clustal Omega, accurate alignment of very large numbers of sequences. Multiple Seq alignment methods. :105–16
Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T et al (2016) ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res 44(W1):W344–W50
Consortium U (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47(D1):D506–D15
Zhao C, Xiao Y, Ling S, Pei Y, Ren J (2021) Structure of collagen. Methods in molecular biology. (Clifton NJ) 2347:17–25
Delbaere S, Dhooge T, Syx D, Petit F, Goemans N, Destrée A et al (2020) Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers–Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet Sci 22(1):112–123
Izu Y, Birk DE (2023) Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease. Front Cell Dev Biology 11:1129000
Yonekawa T, Nishino I (2015) Ullrich congenital muscular dystrophy: clinicopathological features, natural history and pathomechanism (s). J Neurol Neurosurg Psychiatry 86(3):280–287
Di Martino A, Cescon M, D’Agostino C, Schilardi F, Sabatelli P, Merlini L et al (2023) Collagen VI in the Musculoskeletal System. Int J Mol Sci 24(6):5095
Funding
The research project has been financially supported by Golestan University of Medical Sciences (Grant Number: 111672).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naghipoor, K., Khosravi, T. & Oladnabi, M. Whole exome sequencing identifies a novel variant in the COL12A1 gene in a family with Ullrich congenital muscular dystrophy 2. Mol Biol Rep 50, 7427–7435 (2023). https://doi.org/10.1007/s11033-023-08644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08644-6