Abstract
Objective
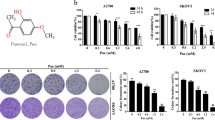
Our previous studies showed that naringin (Nar) can effectively reverse the cisplatin resistance of ovarian cancer cells. This study aims to explore the potential mechanism by which Nar reverses cisplatin resistance in ovarian cancer.
Methods
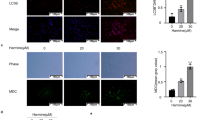
The proliferative activity of cells was evaluated using CCK8 and cell clone formation assays. Autophagic flux in cells was evaluated via LC3B immunofluorescence and monodansylcadaverine (MDC) staining. The expression levels of autophagy, endoplasmic reticulum (ER) stress, and apoptosis-related proteins were detected via Western blotting. Autophagy and ER stress were regulated using siATG5, siLC3B, rapamycin (Rap), chloroquine (CQ), 4-phenylbutyric acid (4-PBA), and thapsigargin (TG). siATG5 and siLC3B are short interfering RNAs (siRNAs) used to knock down the expression of ATG5 and LC3B genes, respectively.
Results
Nar inhibited autophagy in SKOV3/DDP cells by activating the PI3K/AKT/mTOR pathway. And Nar increased the levels of ER stress-related proteins, namely, P-PERK, GRP78, and CHOP, and promoted apoptosis in SKOV3/DDP cells. Moreover, treatment with the inhibitor of ER stress alleviated apoptosis induced by Nar in SKOV3/DDP cells. In addition, compared to cisplatin or naringin alone, the combination of Nar and cisplatin significantly reduced the proliferative activity of SKOV3/DDP cells. And siATG5, siLC3B, CQ or TG pretreatment further inhibited the proliferative activity of SKOV3/DDP cells. Conversely, Rap or 4-PBA pretreatment alleviated the cell proliferation inhibition caused by Nar combined with cisplatin.
Conclusion
Nar not only inhibited the autophagy in SKOV3/DDP cells by regulating the PI3K/AKT/mTOR signalling pathway, but also promoted apoptosis in SKOV3/DDP cells by targeting ER stress. Nar can reverse the cisplatin resistance in SKOV3/DDP cells through these two mechanisms.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kuroki L, Guntupalli SR (2020) Treatment of epithelial ovarian cancer. BMJ. https://doi.org/10.1136/bmj.m3773
Lheureux S, Gourley C, Vergote I et al (2019) Epithelial ovarian cancer. Lancet 393(10177):1240–1253. https://doi.org/10.1016/S0140-6736(18)32552-2
Alam F, Mohammadin K, Shafique Z et al (2022) Citrus flavonoids as potential therapeutic agents: a review. Phytother Res 36(4):1417–1441. https://doi.org/10.1002/ptr.7261
Ghanbari-Movahed M, Jackson G, Farzaei MH et al (2021) A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front Pharmacol 12:639840. https://doi.org/10.3389/fphar.2021.639840
Stabrauskiene J, Kopustinskiene DM, Lazauskas R et al (2022) Naringin and naringenin: their mechanisms of action and the potential anticancer activities. Biomedicines. https://doi.org/10.3390/biomedicines10071686
Zhu H, Gao J, Wang L et al (2018) In vitro study on reversal of ovarian cancer cell resistance to cisplatin by naringin via the nuclear factor-kappaB signaling pathway. Exp Ther Med 15(3):2643–2648. https://doi.org/10.3892/etm.2018.5695
Zhu H, Zou X, Lin S et al (2020) Effects of naringin on reversing cisplatin resistance and the Wnt/beta-catenin pathway in human ovarian cancer SKOV3/CDDP cells. J Int Med Res 48(10):1219687421. https://doi.org/10.1177/0300060519887869
Russell RC, Guan KL (2022) The multifaceted role of autophagy in cancer. EMBO J 41(13):e110031. https://doi.org/10.15252/embj.2021110031
Sui X, Chen R, Wang Z et al (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838. https://doi.org/10.1038/cddis.2013.350
Yang L, Xie HJ, Li YY et al (2022) Molecular mechanisms of platinumbased chemotherapy resistance in ovarian cancer (Review). Oncol Rep. https://doi.org/10.3892/or.2022.8293
Circu M, Cardelli J, Barr MP et al (2017) Modulating lysosomal function through lysosome membrane permeabilization or autophagy suppression restores sensitivity to cisplatin in refractory non-small-cell lung cancer cells. PLoS ONE 12(9):e184922. https://doi.org/10.1371/journal.pone.0184922
Yu H, Su J, Xu Y et al (2011) p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur J Cancer 47(10):1585–1594. https://doi.org/10.1016/j.ejca.2011.01.019
Zhou Y, Wang C, Ding J et al (2022) miR-133a targets YES1 to reduce cisplatin resistance in ovarian cancer by regulating cell autophagy[J]. Cancer Cell Int 22(1):15. https://doi.org/10.1186/s12935-021-02412-x
Pavel M, Rubinsztein DC (2017) Mammalian autophagy and the plasma membrane. FEBS J 284(5):672–679. https://doi.org/10.1111/febs.13931
Tran S, Fairlie WD, Lee EF (2021) BECLIN1: protein structure, function and regulation. Cells. https://doi.org/10.3390/cells10061522
Kumar AV, Mills J, Lapierre LR (2022) Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front Cell Dev Biol 10:793328. https://doi.org/10.3389/fcell.2022.793328
Braakman I, Hebert DN (2013) Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol 5(5):a13201. https://doi.org/10.1101/cshperspect.a013201
Wiseman RL, Mesgarzadeh JS, Hendershot LM (2022) Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol Cell 82(8):1477–1491. https://doi.org/10.1016/j.molcel.2022.03.025
Balch WE, Morimoto RI, Dillin A et al (2008) Adapting proteostasis for disease intervention. Science 319(5865):916–919. https://doi.org/10.1126/science.1141448
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21(8):421–438. https://doi.org/10.1038/s41580-020-0250-z
Balgoma D, Kullenberg F, Calitz C et al (2021) Anthracyclins increase PUFAs: potential implications in ER stress and cell death. Cells. https://doi.org/10.3390/cells10051163
Sovolyova N, Healy S, Samali A et al (2014) Stressed to death—mechanisms of ER stress-induced cell death. Biol Chem 395(1):1–13. https://doi.org/10.1515/hsz-2013-0174
Li Y, Guo Y, Tang J et al (2014) New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 46(8):629–640. https://doi.org/10.1093/abbs/gmu048
Guo H, Ren F, Zhang L et al (2016) Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Mol Med Rep 13(3):2791–2800. https://doi.org/10.3892/mmr.2016.4845
Wang L, Hu R, Dai A (2022) Curcumin increased the sensitivity of non-small-cell lung cancer to cisplatin through the endoplasmic reticulum stress pathway. Evid Based Complement Alternat Med 2022:6886366. https://doi.org/10.1155/2022/6886366
Liu N, Xu Y, Sun JT et al (2013) The BH3 mimetic S1 induces endoplasmic reticulum stress-associated apoptosis in cisplatin-resistant human ovarian cancer cells although it activates autophagy. Oncol Rep 30(6):2677–2684. https://doi.org/10.3892/or.2013.2771
Xu Y, Yu H, Qin H et al (2012) Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett 314(2):232–243. https://doi.org/10.1016/j.canlet.2011.09.034
Funding
This study was funded by National Natural Science Foundation of China (Grant numbers [82060477]), Natural Science Foundation of Jiangxi Province (Grant numbers [20202BAB206054]), Science and Technology Plan of Jiangxi Provincial Health Commission in 2021 (Grant numbers [202120072]), Nanchang Advantage science and technology Innovation team construction Plan project (Hongke Zi (2019) No.228-15), Nanchang high-level scientific and technological innovation talents“Double Hundred Plan”project (Hongke Zi (2021) No. 156-29), General project of Traditional Chinese Medicine Science and Technology Plan of Jiangxi Province in 2021 (Grant numbers [2021B666]), Nanchang Science and Technology Bureau Science and technology support project (Hongke Zi (2019) No.258-6), and Nanchang City medical health science and technology support project key project (Hongke Zi (2021) No.129-1) and Jiangxi Province Graduate Students Innovation Special Fund project in 2022(Grant numbers [YC2022-B069]).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation,were performed by [JZ], [XC] and [XY] data collection and analysis were performed by [JZ], [SL], [XZ] and [YL]. Editing, visualization, supervision, funding acquisition, project administration was performed by [HZ], [JG]. The first draft of the manuscript was written by [JZ] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Lin, S., Zou, X. et al. Mechanisms of autophagy and endoplasmic reticulum stress in the reversal of platinum resistance of epithelial ovarian cancer cells by naringin. Mol Biol Rep 50, 6457–6468 (2023). https://doi.org/10.1007/s11033-023-08558-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08558-3