Abstract
Background
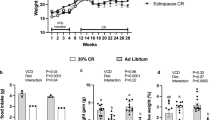
Loss of ovarian function is associated with increased visceral fat. In this study, we aimed to study the effects of caloric restriction (CR) on metabolism in ovariectomized mice.
Methods and results
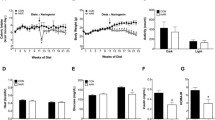
Female, 8–12-month-old mice were divided into three groups: OVX (ovariectomized mice), OVXR (40% CR) and Sham. CR increased insulin sensitivity and glucose tolerance. AMPK phosphorylation was observed in the liver of OVXR mice. CR also increased hepatic cholesterol and triglyceride levels. The reductions in the level of TBARS in the serum and liver and of H2O2 in the liver of OVXR mice suggested alterations in the redox state of the liver. Although expression of catalase protein was reduced by CR, expression of superoxide dismutase was not altered by CR. Although interleukin IL-6 and IL-10 levels in OVXR mice were similar to those in Sham mice, macrophage infiltration was reduced in OVXR mice. OVXR mice had increased sirtuin1 levels and decreased sirtuin3 levels in the liver.
Conclusions
In conclusion, CR improved the condition of ovariectomized mice by reducing adiposity and increasing insulin sensitivity and glucose tolerance through a mechanism that may involve AMPK.






Similar content being viewed by others
Data Availability
Data generated or analyzed during this study are available form the corresponding author upon reasonable request.
References
Qian SW, Liu Y, Wang J, Nie JC, Wu MY, Tang Y et al (2016) BMP4 cross-talks with Estrogen/ERalpha signaling to regulate adiposity and glucose metabolism in females. EBioMedicine 11:91–100. https://doi.org/10.1016/j.ebiom.2016.07.034
Lobo RA, Gompel A (2022) Management of menopause: a view towards prevention. Lancet Diabetes Endocrinol 10:457–470. https://doi.org/10.1016/S2213-8587(21)00269-2
Cerdas Perez S (2023) Menopause and diabetes. Climacteric:1–6. https://doi.org/10.1080/13697137.2023.2184252
Fenton A, Smart C, Goldschmidt L, Price V, Scott J (2023) Fat mass, weight and body shape changes at menopause - causes and consequences: a narrative review. Climacteric 1–7. https://doi.org/10.1080/13697137.2023.2178892
Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D et al (2012) Understanding weight gain at menopause. Climacteric 15:419–429. https://doi.org/10.3109/13697137.2012.707385
Choi SB, Jang JS, Park S (2005) Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 146:4786–4794. https://doi.org/10.1210/en.2004-1653
Hamilton DJ, Minze LJ, Kumar T, Cao TN, Lyon CJ, Geiger PC et al (2016) Estrogen receptor alpha activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Physiol Rep 4. https://doi.org/10.14814/phy2.12913
Habibi P, Ahmadiasl N, Nourazarian A, Yousefi H (2022) Swimming exercise improves SIRT1, NF-kappaB, and IL-1beta protein levels and pancreatic tissue injury in ovariectomized diabetic rats. Horm Mol Biol Clin Investig 43:345–352. https://doi.org/10.1515/hmbci-2021-0069
Kim B, Park ES, Lee JS, Suh JG (2023) Outbred mice with Streptozotocin-Induced Diabetes Show sex differences in glucose metabolism. Int J Mol Sci 24. https://doi.org/10.3390/ijms24065210
Guo J, Huang X, Dou L, Yan M, Shen T, Tang W et al (2022) Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther 7:391. https://doi.org/10.1038/s41392-022-01251-0
Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS et al (2017) Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8:14063. https://doi.org/10.1038/ncomms14063
Zhang S, Sun S, Wei X, Zhang M, Chen Y, Mao X et al (2022) Short-term moderate caloric restriction in a high-fat diet alleviates obesity via AMPK/SIRT1 signaling in white adipocytes and liver. Food Nutr Res 66. https://doi.org/10.29219/fnr.v66.7909
Lawniczak A, Wronska A, Wierzbicki P, Kmiec Z (2022) Aging and short-term calorie restriction differently affect the cardiac and skeletal muscle expression of genes regulating energy substrate utilization in male rats. Biogerontology 23:325–340. https://doi.org/10.1007/s10522-022-09965-y
Longo VD, Anderson RM (2022) Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell 185:1455–1470. https://doi.org/10.1016/j.cell.2022.04.002
Palmer AK, Jensen MD (2022) Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest 132. https://doi.org/10.1172/JCI158451
de Faria SN, Pimentel MS, Helaehil VE, Bertolo JV, Santos MC, da Silva-Neto NTH PV et al (2022) Caloric restriction overcomes pre-diabetes and hypertension induced by a high fat diet and renal artery stenosis. Mol Biol Rep 49:5883–5895. https://doi.org/10.1007/s11033-022-07370-9
Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL, Liu YY et al (2022) The sirtuin family in health and disease. Signal Transduct Target Ther 7:402. https://doi.org/10.1038/s41392-022-01257-8
Kim JY, Mondaca-Ruff D, Singh S, Wang Y (2022) SIRT1 and autophagy: implications in Endocrine Disorders. Front Endocrinol (Lausanne) 13:930919. https://doi.org/10.3389/fendo.2022.930919
Tozzi R, Cipriani F, Masi D, Basciani S, Watanabe M, Lubrano C et al (2022) Ketone bodies and SIRT1, synergic epigenetic regulators for Metabolic Health: a narrative review. Nutrients 14. https://doi.org/10.3390/nu14153145
Wei X, Wei C, Tan Y, Dong X, Yang Z, Yan J et al (2023) Both prolonged high-fat diet consumption and calorie restriction boost hepatic NAD + metabolism in mice. J Nutr Biochem 115:109296. https://doi.org/10.1016/j.jnutbio.2023.109296
Opstad TB, Sundfor T, Tonstad S, Seljeflot I (2021) Effect of intermittent and continuous caloric restriction on Sirtuin1 concentration depends on sex and body mass index. Nutr Metab Cardiovasc Dis 31:1871–1878. https://doi.org/10.1016/j.numecd.2021.03.005
Ding RB, Bao J, Deng CX (2017) Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci 13:852–867. https://doi.org/10.7150/ijbs.19370
McGinnis CD, Jennings EQ, Harris PS, Galligan JJ, Fritz KS (2022) Biochemical mechanisms of Sirtuin-Directed protein acylation in hepatic pathologies of mitochondrial dysfunction. Cells 11. https://doi.org/10.3390/cells11132045
Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B et al (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44:177–190. https://doi.org/10.1016/j.molcel.2011.07.019
Pinteric M, Podgorski II, Hadzija MP, Bujak IT, Dekanic A, Bagaric R et al (2020) Role of Sirt3 in Differential Sex-Related responses to a High-Fat Diet in mice. Antioxid (Basel) 9. https://doi.org/10.3390/antiox9020174
Ingram DK, de Cabo R (2017) Calorie restriction in rodents: caveats to consider. Ageing Res Rev 39:15–28. https://doi.org/10.1016/j.arr.2017.05.008
Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI et al (2010) Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3:525–534. https://doi.org/10.1242/dmm.006239
Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V et al (1989) Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab 68:374–378. https://doi.org/10.1210/jcem-68-2-374
Lo S, Russell JC, Taylor AW (1970) Determination of glycogen in small tissue samples. J Appl Physiol 28:234–236. https://doi.org/10.1152/jappl.1970.28.2.234
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Serra CA, Dos Reis AF, Calsa B, Bueno CS, Helaehil JV, de Souza SAR et al (2022) Quercetin prevents insulin dysfunction in hypertensive animals. J Diabetes Metab Disord 21:407–417. https://doi.org/10.1007/s40200-022-00987-4
Corezola do Amaral ME, Kravets V, Dwulet JM, Farnsworth NL, Piscopio R, Schleicher WE et al (2020) Caloric restriction recovers impaired beta-cell-beta-cell gap junction coupling, calcium oscillation coordination, and insulin secretion in prediabetic mice. Am J Physiol Endocrinol Metab 319:E709–E20. https://doi.org/10.1152/ajpendo.00132.2020
Suchacki KJ, Thomas BJ, Ikushima YM, Chen KC, Fyfe C, Tavares AAS et al (2023) The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. Elife 12. https://doi.org/10.7554/eLife.88080
Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A et al (2005) Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54:1331–1339. https://doi.org/10.2337/diabetes.54.5.1331
Kim J, Lee H, Lim J, Lee H, Yoon S, Shin SS et al (2017) The lemon balm extract ALS-L1023 inhibits obesity and nonalcoholic fatty liver disease in female ovariectomized mice. Food Chem Toxicol 106:292–305. https://doi.org/10.1016/j.fct.2017.05.059
Lei Z, Wu H, Yang Y, Hu Q, Lei Y, Liu W et al (2021) Ovariectomy impaired hepatic glucose and lipid homeostasis and altered the gut microbiota in mice with different diets. Front Endocrinol (Lausanne) 12:708838. https://doi.org/10.3389/fendo.2021.708838
Boudaba N, Marion A, Huet C, Pierre R, Viollet B, Foretz M (2018) AMPK Re-Activation suppresses hepatic steatosis but its downregulation does not promote fatty Liver Development. EBioMedicine 28:194–209. https://doi.org/10.1016/j.ebiom.2018.01.008
Foretz M, Even PC, Viollet B (2018) AMPK Activation reduces hepatic lipid content by increasing Fat Oxidation in vivo. Int J Mol Sci 19. https://doi.org/10.3390/ijms19092826
Camargo TF, Zanesco AM, Pacher KAS, Andrade TAM, Alves AA, do Amaral MEC (2020) Physiological profile regulation during weight gain and loss by ovariectomized females: importance of SIRT1 and SIRT4. Am J Physiol Endocrinol Metab 319:E769–E78. https://doi.org/10.1152/ajpendo.00465.2019
Shin JW, Lee E, Han S, Choe SA, Jeon OH (2022) Plasma proteomic signature of Cellular Senescence and markers of Biological Aging among Postmenopausal Women. Rejuvenation Res 25:141–148. https://doi.org/10.1089/rej.2022.0024
Shi X, Jiang J, Hong R, Xu F, Dai S (2023) Circulating IGFBP-3 and interleukin 6 as predictors of osteoporosis in Postmenopausal Women: a cross-sectional study. Mediators Inflamm 2023:2613766. https://doi.org/10.1155/2023/2613766
Vural P, Canbaz M, Akgul C (2006) Effects of menopause and postmenopausal tibolone treatment on plasma TNFalpha, IL-4, IL-10, IL-12 cytokine pattern and some bone turnover markers. Pharmacol Res 53:367–371. https://doi.org/10.1016/j.phrs.2006.01.005
Corral J, Miralles JM, Garcia-Pascual IJ, Corrales JJ, Garcia-Sastre A, Villar E (1992) Increased serum N-acetyl-beta-D-glucosaminidase and alpha-D-mannosidase activities in obese subjects. Clin Investig 70:880–884. https://doi.org/10.1007/BF00180432
Suh JS, Cho KS, Kim SK, Kim SH, Cho WK, Jung MH et al (2022) High Glycated Hemoglobin instead of high body Mass Index might increase the urine N-Acetyl-beta-D-glucosaminidase con-centration in children and adolescents with diabetes Mellitus. Life (Basel) 12. https://doi.org/10.3390/life12060879
Sasaki Y, Ikeda Y, Miyauchi T, Uchikado Y, Akasaki Y, Ohishi M (2020) Estrogen-SIRT1 Axis plays a pivotal role in protecting arteries against Menopause-Induced Senescence and Atherosclerosis. J Atheroscler Thromb 27:47–59. https://doi.org/10.5551/jat.47993
Pinteric M, Podgorski II, Popovic Hadzija M, Tartaro Bujak I, Tadijan A, Balog T et al (2021) Chronic high Fat Diet Intake impairs hepatic metabolic parameters in Ovariectomized Sirt3 KO mice. Int J Mol Sci 22. https://doi.org/10.3390/ijms22084277
Ltaif M, Gargouri M, Soussi A (2021) Protective Effects of A. sativa against oxidative Stress-Induced Liver damage in Ovariectomized mice. Biomed Res Int 2021:5577498. https://doi.org/10.1155/2021/5577498
Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C et al (2011) Inflammation, oxidative stress, and obesity. Int J Mol Sci 12:3117–3132. https://doi.org/10.3390/ijms12053117
Mittal PC, Kant R (2009) Correlation of increased oxidative stress to body weight in disease-free post menopausal women. Clin Biochem 42:1007–1011. https://doi.org/10.1016/j.clinbiochem.2009.03.019
Acknowledgements
We thank Ana Cristina Pires Menegheti, Bruno Alves Cia, Lucas Orzari, Mateus Eduardo Bortolanza da Silva, and Renata Barbieri for technical assistance.
Funding
This work was supported by the Herminio Ometto Foundation.
Author information
Authors and Affiliations
Contributions
Sapatini LRL: Data curation, Investigation, Visualization, Writing – original draft. Calsa B: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, review and editing. Marim LJ: Investigation. Helaehil JV: Data curation, Investigation. Amaral MEC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethics approval
The project was approved by the Committee on Ethics in Animal Use (CEUA) under protocol #009/2020 of University Center of Hermínio Ometto Foundation, Araras, SP, Brazil.
Preprint or repository availability
bioRxiv preprint https://doi.org/10.1101/2023.02.09.527836.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sapatini, L.R.L., Calsa, B., Marim, L.J. et al. Caloric restriction prevents inflammation and insulin dysfunction in middle-aged ovariectomized mice. Mol Biol Rep 50, 5675–5685 (2023). https://doi.org/10.1007/s11033-023-08508-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08508-z