Abstract
Background
Retinal pigment epithelium (RPE) cells are potential targets for treating retinal detachment (RD) and proliferative vitreoretinopathy (PVR), considering the importance of neuroprotection and epithelial-mesenchymal transition (EMT) of RPE in these conditions. This study investigated the effect of human Wharton’s jelly mesenchymal stem cell secretome (WJMSC-S) on the expression of genes involved in both neuroprotection and EMT in RPE cells in vitro (TRKB, MAPK, PI3K, BDNF, and NGF).
Methods
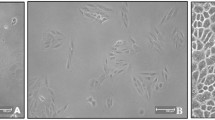
RPE cells from passages 5–7 were treated with WJMSC-S (or the vehicle culture medium as control) for 24 h at 37◦C and subsequently subjected to RNA extraction and cDNA synthesis. Gene expression level was evaluated using real-time PCR in the treated versus control cells.
Results
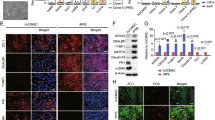
The results of our study showed that WJMSC-S led to a significant downregulation in three out of five studied gene expression (MAPK, TRKB, and NGF), and simultaneously, remarkably upregulated the expression of the BDNF gene.
Conclusions
According to the present data, WJMSC-S can affect the EMT and neuroprotection processes at the mRNA level by suppressing EMT and promoting neuroprotection in RPE cells. This finding may have positive clinical implications in the context of RD and PVR.





Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Idrees S, Sridhar J, Kuriyan AE (2019) Proliferative vitreoretinopathy: a review. Int Ophthalmol Clin 59(1):221
Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A (2014) Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res 40:16–34
Mudhar HS (2020) A brief review of the histopathology of proliferative vitreoretinopathy (PVR). Eye 34(2):246–250
Charteris DG (2020) Proliferative vitreoretinopathy: revised concepts of pathogenesis and adjunctive treatment. Eye 34(2):241–245
Chiba C (2014) The retinal pigment epithelium: an important player of retinal disorders and regeneration. Exp Eye Res 123:107–114
Hu D-N, Gentile R, McCormick S, Yang P, Muldoon T, Lin H (2017) Role of RPE cells in pathogenesis of proliferative vitreoretinopathy and age-related macular degeneration: cell culture study of surgical excised pre-and sub-retinal membranes. J Clin Ophthalmol Eye Disord 1(1):1002
Cook B, Lewis GP, Fisher SK, Adler R (1995) Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 36(6):990–996
Ghazi N, Green W (2002) Pathology and pathogenesis of retinal detachment. Eye 16(4):411–421
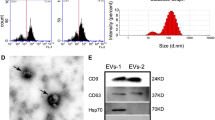
Sanie-Jahromi F, Nowroozzadeh MH, Khodabandeh Z, Soheili Z-S, Khajehahmadi Z, Emadi Z et al (2021) Effects of the secretome of human Wharton’s jelly mesenchymal stem cells on the proliferation and apoptosis gene expression of the retinal pigmented epithelium. Exp Eye Res 205:108528
Stefańska K, Ożegowska K, Hutchings G, Popis M, Moncrieff L, Dompe C et al (2020) Human Wharton’s jelly—cellular specificity, stemness potency, animal models, and current application in human clinical trials. J Clin Med 9(4):1102
Troyer DL, Weiss ML (2008) Concise review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3):591–599
Carlin R, Davis D, Weiss M, Schultz B, Troyer D (2006) Expression of early transcription factors Oct-4, Sox-2 and nanog by porcine umbilical cord (PUC) matrix cells. Reproductive Biology and Endocrinology 4(1):1–13
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M et al (2009) Isolation and characterization of Oct-4+/HLA-G + mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol 131:267–282
Vangsness CT Jr, Sternberg H, Harris L (2015) Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy: The Journal of Arthroscopic & Related Surgery 31(9):1836–1843
Sobolewski K, Małkowski A, Bańkowski E, Jaworski S (2005) Wharton’s jelly as a reservoir of peptide growth factors. Placenta 26(10):747–752
Gupta A, El-Amin SF, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N (2020) Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J Orthop Surg Res 15(1):1–9
Main BJ, Valk JA, Maffulli N, Rodriguez HC, Gupta M, Stone IW et al (2020) Umbilical cord-derived Wharton’s jelly for regenerative medicine applications in orthopedic surgery: a systematic review protocol. J Orthop Surg Res 15(1):1–5
Tian H, Xu J-Y, Tian Y, Cao Y, Lian C, Ou Q et al (2018) A cell culture condition that induces the mesenchymal-epithelial transition of dedifferentiated porcine retinal pigment epithelial cells. Exp Eye Res 177:160–172
Harrell CR, Fellabaum C, Arsenijevic A, Markovic BS, Djonov V, Volarevic V (2019) Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of glaucoma. Stem Cells Int. ;2019
Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V (2019) Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8(5):467
Kumar P, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K, Verma RS (2019) The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev 46:1–9
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017) Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 18(9):1852
Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL (2013) TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci 14(5):10122–10142
Kupferman M, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T et al (2010) TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene 29(14):2047–2059
Ricci A, De Vitis C, Noto A, Fattore L, Mariotta S, Cherubini E et al (2013) TrkB is responsible for EMT transition in malignant pleural effusions derived cultures from adenocarcinoma of the lung. Cell Cycle 12(11):1696–1703
Bao W, Qiu H, Yang T, Luo X, Zhang H, Wan X (2013) Upregulation of TrkB promotes epithelial-mesenchymal transition and anoikis resistance in endometrial carcinoma. PLoS ONE 8(7):e70616
Smit MA, Geiger TR, Song J-Y, Gitelman I, Peeper DS (2009) A twist-snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 29(13):3722–3737
Kupferman M, Zhou G, Ju J, El-Naggar A, Yu D, Myers J (2008) Critical role of TrkB for invasion and EMT in HNSCC. Cancer Res 68(9Supplement):5370
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75(1):50–83
Gui T, Sun Y, Shimokado A, Muragaki Y (2012) The roles of mitogen-activated protein kinase pathways in TGF-β-induced epithelial-mesenchymal transition. J Signal Transduct. ;2012
Huang M, Wang YP, Zhu LQ, Cai Q, Li HH, Yang HF (2016) MAPK pathway mediates epithelial-mesenchymal transition induced by paraquat in alveolar epithelial cells. Environ Toxicol 31(11):1407–1414
Xu M, Wang S, Wang Y, Wu H, Frank JA, Zhang Z et al (2018) Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochim Biophys Acta Mol Basis Dis BBA-MOL BASIS DIS 1864(11):3605–3617
Chang Y-H, Vuong C-K, Ngo N-H, Yamashita T, Ye X, Futamura Y et al (2022) Extracellular vesicles derived from Wharton’s Jelly mesenchymal stem cells inhibit the tumor environment via the miR-125b/HIF1α signaling pathway. Sci Rep 12(1):13550
Uzunhan Y, Bernard O, Marchant D, Dard N, Vanneaux V, Larghero J et al (2016) Mesenchymal stem cells protect from hypoxia-induced alveolar epithelial-mesenchymal transition. Am J Physiology-Lung Cell Mol Physiol 310(5):L439–L51
Shin S, Lee J, Kwon Y, Park K-S, Jeong J-H, Choi S-J et al (2021) Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and wharton’s jelly. Int J Mol Sci 22(2):845
Shibata S, Hayashi R, Okubo T, Kudo Y, Baba K, Honma Y et al (2019) The secretome of adipose-derived mesenchymal stem cells attenuates epithelial–mesenchymal transition in human corneal epithelium. Regen Ther 11:114–122
Liu S, Fan M, Xu J-X, Yang L-J, Qi C-C, Xia Q-R et al (2022) Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflamm 19(1):35
Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factors signaling, neuroprotection, and nerual repair. Annu Rev Neurosci 24:1217
Li B, Ning B, Yang F, Guo C (2022) Nerve growth factor promotes retinal neurovascular unit repair: a review.Curr Eye Res. :1–11
Fudalej E, Justyniarska M, Kasarełło K, Dziedziak J, Szaflik JP, Cudnoch-Jędrzejewska A (2021) Neuroprotective factors of the retina and their role in promoting survival of retinal ganglion cells: a review. Ophthalmic Res 64(3):345–355
Lin C, Ren Z, Yang X, Yang R, Chen Y, Liu Z et al (2020) Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer lett 472:81–96
Acknowledgements
The authors would like to thank Dr. Zahra Khodabandeh (Stem Cells Technology Research Center, Shiraz University of Medical Science) for her kind assistance in this study.
Funding
This study was supported by Shiraz University of Medical Science (Grant# 24961).
Author information
Authors and Affiliations
Contributions
F.S. and MH.N. conceived and designed the study, analyzed the data; F.S. performed the experimental analysis; F.S., MH.N., and Sh. Gh. wrote the main manuscript and revised the final version. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1400.552), and informed written consent was obtained from donors and participants.
Consent for publication
Not applicable.
Conflict of interest
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nowroozzadeh, M.H., Ghazanfari, S. & Sanie-Jahromi, F. Human Wharton’s Jelly Mesenchymal Stem Cell Secretome Modifies the Processes of Neuroprotection and Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium at Transcriptional Level. Mol Biol Rep 50, 5725–5732 (2023). https://doi.org/10.1007/s11033-023-08496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08496-0