Abstract
Background
Inflammatory bowel disease (IBD) is a global health problem and there are few cell models for IBD at present. To culture a human fetal colon (FHC) cell line in vitro and establish an FHC cell inflammation model that meets the requirements for high expression of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).
Methods and results
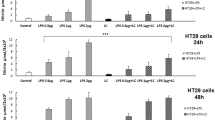
FHC cells were cultured with various concentrations of Escherichia coli lipopolysaccharide (LPS) in appropriate media for 0.5, 1, 2, 4, 8, 16 and 24 h to stimulate an inflammatory reaction. The viability of FHC cells was detected by a Cell Counting Kit-8 (CCK-8) assay. The transcriptional levels and protein expression changes of IL-6 and TNF-α in FHC cells were detected by Quantitative Real‑Time Polymerase Chain Reaction (qRT-PCR) and Enzyme‑Linked Immunosorbent Assay (ELISA), respectively. Appropriate stimulation conditions were selected (i.e., LPS concentration and treatment time), based on changes in cell survival rate, and IL-6 and TNF-α expression levels. An LPS concentration higher than 100 µg/mL or a treatment time longer than 24 h resulted in morphological changes and decreased cell survival. By contrast, expression levels of IL-6 and TNF-α significantly increased within 24 h when LPS concentration lower than 100 µg/mL and peaked at 2 h, whilst maintaining cell morphology and viability in FHC cells.
Conclusion
The treatment of FHC cells with 100 µg/mL LPS within 24 h was optimal in terms of stimulating IL-6 and TNF-α expression.




Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369(9573):1641–1657. https://doi.org/10.1016/s0140-6736(07)60751-x
Lakatos L, Mester G, Erdelyi Z, Balogh M, Szipocs I, Kamaras G, Lakatos PL (2004) Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977–2001. World J Gastroenterol 10(3):404–409. https://doi.org/10.3748/wjg.v10.i3.404
Axelrad JE, Lichtiger S, Yajnik V (2016) Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 22(20):4794–4801. https://doi.org/10.3748/wjg.v22.i20.4794
Uranga JA, López-Miranda V, Lombó F, Abalo R (2016) Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol Rep 68(4):816–826. https://doi.org/10.1016/j.pharep.2016.05.002
Uciechowski P, Dempke WCM (2020) Interleukin-6: a masterplayer in the Cytokine Network. Oncology 98(3):131–137. https://doi.org/10.1159/000505099
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374(Pt 1):1–20. https://doi.org/10.1042/bj20030407
Bradley JR (2008) TNF-mediated inflammatory disease. J Pathol 214(2):149–160. https://doi.org/10.1002/path.2287
Waldner MJ, Neurath MF (2014) Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol 26(1):75–79. https://doi.org/10.1016/j.smim.2013.12.003
Wine E, Mack DR, Hyams J, Otley AR, Markowitz J, Crandall WV, Turner D (2013) Interleukin-6 is associated with steroid resistance and reflects disease activity in severe pediatric ulcerative colitis. J Crohns Colitis 7(11):916–922. https://doi.org/10.1016/j.crohns.2012.12.012
Ye M, Joosse ME, Liu L, Sun Y, Dong Y, Cai C, Li X (2020) Deletion of IL-6 exacerbates colitis and induces systemic inflammation in IL-10-Deficient mice. J Crohns Colitis 14(6):831–840. https://doi.org/10.1093/ecco-jcc/jjz176
Aardoom MA, Veereman G, de Ridder L (2019) A review on the use of Anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci 20(10). https://doi.org/10.3390/ijms20102529
Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Barrett TA (2012) p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol 181(4):1306–1315. https://doi.org/10.1016/j.ajpath.2012.06.016
Guo C, Guo D, Fang L, Sang T, Wu J, Guo C, Wang X (2021) Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr Polym 267:118231. https://doi.org/10.1016/j.carbpol.2021.118231
Marafini I, Monteleone I, Dinallo V, Di Fusco D, De Simone V, Laudisi F, Monteleone G (2017) CCL20 is negatively regulated by TGF-β1 in intestinal epithelial cells and reduced in Crohn’s Disease Patients with a successful response to Mongersen, a Smad7 antisense oligonucleotide. J Crohns Colitis 11(5):603–609. https://doi.org/10.1093/ecco-jcc/jjw191
Kim JT, Napier DL, Kim J, Li C, Lee EY, Weiss HL, Evers BM (2021) Ketogenesis alleviates TNFα-induced apoptosis and inflammatory responses in intestinal cells. Free Radic Biol Med 172:90–100. https://doi.org/10.1016/j.freeradbiomed.2021.05.032
Pierdomenico M, Palone F, Cesi V, Vitali R, Mancuso AB, Cucchiara S, Stronati L (2018) Transcription factor ZNF281: a novel player in intestinal inflammation and fibrosis. Front Immunol 9:2907. https://doi.org/10.3389/fimmu.2018.02907
Li P, Wang Y, Luo J, Zeng Q, Wang M, Bai M, Jiang H (2020) Downregulation of OCTN2 by cytokines plays an important role in the progression of inflammatory bowel disease. Biochem Pharmacol 178:114115. https://doi.org/10.1016/j.bcp.2020.114115
Liu S, Xu A, Gao Y, Xie Y, Liu Z, Sun M, Wang X (2021) Graphene oxide exacerbates dextran sodium sulfate-induced colitis via ROS/AMPK/p53 signaling to mediate apoptosis. J Nanobiotechnol 19(1):85. https://doi.org/10.1186/s12951-021-00832-5
Schumann RR (1992) Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: a short review. Res Immunol 143(1):11–15. https://doi.org/10.1016/0923-2494(92)80074-u
Lee MY, Sun KH, Chiang CP, Huang CF, Sun GH, Tsou YC, Tang SJ (2015) Nitric oxide suppresses LPS-induced inflammation in a mouse asthma model by attenuating the interaction of IKK and Hsp90. Exp Biol Med (Maywood) 240(4):498–507. https://doi.org/10.1177/1535370214554880
Seehase S, Lauenstein HD, Schlumbohm C, Switalla S, Neuhaus V, Förster C, Knauf S (2012) LPS-induced lung inflammation in marmoset monkeys - an acute model for anti-inflammatory drug testing. PLoS ONE 7(8):e43709. https://doi.org/10.1371/journal.pone.0043709
Sun J, Liang W, Yang X, Li Q, Zhang G (2019) Cytoprotective effects of galacto-oligosaccharides on colon epithelial cells via up-regulating miR-19b. Life Sci 231:116589. https://doi.org/10.1016/j.lfs.2019.116589
Zhang H, Wang Y, Li S, Tang X, Liang R, Yang X (2020) SOCS3 protects against neonatal necrotizing enterocolitis via suppressing NLRP3 and AIM2 inflammasome activation and p65 nuclear translocation. Mol Immunol 122:21–27. https://doi.org/10.1016/j.molimm.2020.03.019
Beaugerie L, Rahier JF, Kirchgesner J (2020) Predicting, preventing, and managing treatment-related complications in patients with inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 18(6):1324–1335e1322. https://doi.org/10.1016/j.cgh.2020.02.009
Umehara Y, Kudo M, Nakaoka R, Kawasaki T, Shiomi M (2006) Serum proinflammatory cytokines and adhesion molecules in ulcerative colitis. Hepatogastroenterology 53(72):879–882
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation (grant no. 82173360), Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University (grant no. 13-003-023), Senior Medical Talents Program of Chongqing for Young and Middle-aged (grant no. 11–020), Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (grant no. 2020GDRC014), China Postdoctoral Science Foundation (grant no. 2022M720606), and Special support for postdoctoral of Chongqing (grant no. 2212013362080033).
Author information
Authors and Affiliations
Contributions
LL and ZM designed the study and performed the experiments, KY and YS collected the data, KY and SL analyzed the data, KY and CL prepared the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, K., Liao, S., Li, C. et al. Establishment of a lipopolysaccharide-induced inflammation model of human fetal colon cells. Mol Biol Rep 50, 5557–5564 (2023). https://doi.org/10.1007/s11033-023-08465-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08465-7