Abstract
Background
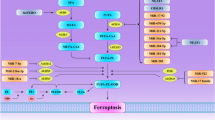
Necroptosis is a controlled form of necrosis which can be stimulated in cases where the apoptosis signal is absent. Necroptosis can be induced by DR family ligands and by various intracellular and extracellular stimuli that triggers the activation of DR family ligands. Necrostatins, which are specific RIP1 antagonists, prevent necroptosis by inhibiting RIP1 kinase, allowing survival and propagation of cells in the presence of DR ligands. Furthermore, there is a mounting evidence that long non-coding RNA (lncRNA) molecules accomplish vital functions in cell death processes such as apoptosis, autophagy, pyroptosis, and necroptosis. Accordingly, here we aimed to decipher the lncRNAs that are involved in the control and maintenance of necroptosis signaling.
Methods and results
Colon cancer cell lines, HT-29 and HCT-116 were used for the study. For the chemical modulation of necroptosis signaling, 5-Fluorouracil, TNF-α and/or Necrostatin-1 were used. Gene expression levels were determined by quantitative real-time PCR. Remarkably, lncRNA P50-associated COX-2 extragenic RNA (PACER) was identified to be suppressed in necroptosis-induced colon cancers, whereas the expression of PACER was restored when necroptosis was suppressed. In addition, no detectable change was observed in HCT-116 colon cancer cells, as these cells lack the expression of RIP3 kinase.
Conclusions
Collectively, current findings clearly imply that PACER have key regulatory roles in the control of necroptotic cell death signaling circuitry. Notably, the tumor promoter activity of PACER might be responsible for the lack of necroptotic death signal in cancer cells. Also, RIP3 kinase seems to be essential component in PACER-associated necroptosis.




Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119
Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22(2):263–268
Chen Y, Ren W, Wang Q, He Y, Ma D, Cai Z (2022) The regulation of necroptosis by ubiquitylation. Apoptosis. https://doi.org/10.1007/s10495-022-01755-8
Galluzzi L, Kepp O, Chan FK-M, Kroemer G (2017) Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol 12:103
Zhou W, Yuan J (2014) Necroptosis in health and diseases. Semin Cell Dev Biol 35:14–23. https://doi.org/10.1016/j.semcdb.2014.07.013
Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H et al (2011) Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477(7364):335–339
Welz P-S, Wullaert A, Vlantis K, Kondylis V, Fernández-Majada V, Ermolaeva M et al (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477(7364):330–334
Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C et al (2014) A positive feedback loop between RIP 3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med 6(8):1062–1074
Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE (2013) Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57(5):1773–1783
Bonnet MC, Preukschat D, Welz P-S, van Loo G, Ermolaeva MA, Bloch W et al (2011) The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35(4):572–582
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L et al (2014) RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513(7516):90–94
Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y et al (2016) RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 353(6299):603–608
Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP et al (2015) Activation of necroptosis in multiple sclerosis. Cell Rep 10(11):1836–1849
Ermine K, Yu J, Zhang L (2022) Role of receptor interacting protein (RIP) kinases in cancer. Genes Dis 9(6):1579–1593. https://doi.org/10.1016/j.gendis.2021.10.007
Yin Z, Chen W, Yin J, Sun J, Xie Q, Wu M et al (2021) RIPK1 is a negative mediator in Aquaporin 1-driven triple-negative breast carcinoma progression and metastasis. NPJ Breast Cancer 7(1):53. https://doi.org/10.1038/s41523-021-00261-5
Brown MF, Leibowitz BJ, Chen D, He K, Zou F, Sobol RW et al (2015) Loss of caspase-3 sensitizes colon cancer cells to genotoxic stress via RIP1-dependent necrosis. Cell Death Dis 6(4):1729. https://doi.org/10.1038/cddis.2015.104
Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM et al (2009) The receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. Cancer Res 69(7):2809–2816. https://doi.org/10.1158/0008-5472.Can-08-4079
Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P (2010) Tumor necrosis factor-mediated cell death: to break or to burst, that’s the question. Cell Mol Life Sci 67(10):1567–1579. https://doi.org/10.1007/s00018-010-0283-0
McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB et al (2014) Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci USA 111(31):E3206–E3213. https://doi.org/10.1073/pnas.1407068111
He S, Liang Y, Shao F, Wang X (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA 108(50):20054–20059. https://doi.org/10.1073/pnas.1116302108
Zhang T, Wang Y, Inuzuka H, Wei W (2022) Necroptosis pathways in tumorigenesis. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2022.07.007
Shan B, Pan H, Najafov A, Yuan J (2018) Necroptosis in development and diseases. Genes Dev 32(5–6):327–340
Slack FJ, Chinnaiyan AM (2019) The role of non-coding RNAs in oncology. Cell 179(5):1033–1055. https://doi.org/10.1016/j.cell.2019.10.017
Hoellen F, Kelling K, Dittmer C, Diedrich K, Friedrich M, Thill MJAr (2011) Impact of cyclooxygenase-2 in breast cancer. Anticancer Res 31(12):4359–4367
Sun P, Quan J-C, Wang S, Zhuang M, Liu Z, Guan X et al (2021) lncRNA-PACER upregulates COX-2 and PGE2 through the NF-κB pathway to promote the proliferation and invasion of colorectal-cancer cells. Gastroenterol Rep 9(3):257–268
Jiang N, Zhang X, Gu X, Li X, Shang L (2021) Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discov 7(1):30. https://doi.org/10.1038/s41420-021-00407-1
Shi G, Jia P, Chen H, Bao L, Feng F, Tang H (2018) Necroptosis occurs in osteoblasts during tumor necrosis factor-α stimulation and caspase-8 inhibition. Braz J Med Biol Res. https://doi.org/10.1590/1414-431x20187844
Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M et al (2013) Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci 110(33):E3109–E3118
Krawczyk M, Emerson BM (2014) p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-κB complexes. Elife 3:e01776. https://doi.org/10.7554/eLife.01776
Christofferson DE, Li Y, Yuan J (2014) Control of life-or-death decisions by RIP1 kinase. Annu Rev Physiol 76:129–150
Ofengeim D, Yuan J (2013) Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol 14(11):727–736
Peltzer N, Darding M, Walczak H (2016) Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol 26(6):445–461
Weinlich R, Oberst A, Beere HM, Green DR (2017) Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18(2):127–136
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131(4):669–681
Vince JE, Wong WW-L, Khan N, Feltham R, Chau D, Ahmed AU et al (2007) IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131(4):682–693
Linkermann A, Green DR (2014) Necroptosis. N Engl J Med 370(5):455–465. https://doi.org/10.1056/NEJMra1310050
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4(5):313–321
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al (2000) Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495
Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J et al (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30(6):689–700
Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L et al (2010) c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J 29(24):4198–4209
Moquin DM, McQuade T, Chan FK-M (2013) CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE 8(10):e76841
Cho Y, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123
Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D et al (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343(6177):1357–1360
Wang H, Sun L, Su L, Rizo J, Liu L, Wang L-F et al (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54(1):133–146
Elliott PR, Leske D, Wagstaff J, Schlicher L, Berridge G, Maslen S et al (2021) Regulation of CYLD activity and specificity by phosphorylation and ubiquitin-binding CAP-Gly domains. Cell Rep 37(1):109777
Funding
The present study was supported by the grant from the Adiyaman University (Grand number: SHMYOMAP/2021-0001).
Author information
Authors and Affiliations
Contributions
Esra Bozgeyik: Conceptualization, Methodology, Writing - Original Draft, Supervision, Haydar Bagis: Conceptualization, Writing - Review & Editing, Ibrahim Bozgeyik: Methodology, Writing - Original Draft, Writing - Review & Editing, Sayad Kocahan: Writing - Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bozgeyik, E., Bagis, H., Bozgeyik, I. et al. The roles of long non-coding RNAs in the necroptotic signaling of colon cancer cells. Mol Biol Rep 50, 5021–5028 (2023). https://doi.org/10.1007/s11033-023-08441-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08441-1