Abstract
Background
Teak (Tectona grandis L.) is a forest tree having 2n = 2x = 36 diploid chromosomes. Plants are continually subjected to variety of abiotic stresses due to climate change, which alter their physiological processes and gene expression.
Methods and results
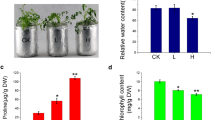
The current study sought to examine the physiological and differential gene expression of teak seedlings exposed to abiotic stresses (150 mM NaCl and 15% PEG-6000). Chlorophyll content, membrane stability index and relative water content were measured at 0, 2, 7 and 12 days after treatment. These parameters were initially numerically reduced, but they were significantly reduced during a longer period of treatment. Seedlings treated with 150 mM NaCl displayed more harmful effect on the plant than other treatments. The results showed that variety of stresses significantly affect the physiology of seedlings because they cause membrane damage, ROS generation, chlorophyll degradation, and reduction in water absorption. The gene expression of treated and control seedlings was also evaluated at 12 days after treatment. Ten stress-related genes were examined for their differential expression using RT-PCR under applied stress. The stress-treated seedlings' leaves showed an up-regulated expression of the genes MYB-3, HSP-1, BI-1 and CS-2.
Conclusion
Up-regulation of the genes confirmed the protective function of these genes in plants under abiotic stress. However, gene expression was affected by treatments, the extent of stress and the species of plant. This study came to the conclusion that physiological parameters could be utilized as marker indices to assess a tree's capability to withstand stress at seedling stage. The up-regulated genes will be further investigated and utilized to validate stress tolerance and susceptible teak seedlings.




Similar content being viewed by others
Abbreviations
- BI:
-
BAX inhibitor
- CRD:
-
Completely random design
- CS:
-
Cellulose synthases
- CT:
-
Threshold cycle
- CXEs:
-
Carboxylesterase
- DAT:
-
Days after treatment
- EC:
-
Electrical conductivity
- HSP:
-
Heat shock protein
- MSI:
-
Membrane stability index
- MYB:
-
Myeloblastosis
- NaCl:
-
Sodium chloride
- NTC:
-
Non-template control
- PAL:
-
Phenylalenine ammonia lyase
- PEG:
-
Polyethylene glycol
- RIN:
-
RNA integrity number
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Real time PCR
- RWC:
-
Relative water content
References
Thakor MC, Fougat RS, Kumar S, Sakure AA (2019) Sequence-related amplified polymorphism (SRAP) analysis of teak (Tectona grandis L.) germplasm. Ecol Genet Genom 12:100041. https://doi.org/10.1016/j.egg.2019.100041
Maisuria HJ, Dhaduk HL, Kumar S, Sakure AA, Thounaojam AT (2022) Teak population structure and genetic diversity in Gujarat India. Curr Plant Biol 32:100267. https://doi.org/10.1016/j.cpb.2022.100267
Katwal R (2003) Teak in India: status, prospects and perspectives. In: Proceedings of the international conference on quality timber products of teak from sustainable forest management. Peechi, pp 2–5.
Rosero C, Argout X, Ruiz M, Teran W (2011) A drought stress transcriptome profiling as the first genomic resource for white teak—Gamhar—(Gmelina arborea Roxb) and related species. BMC Proc 5(7):P178. https://doi.org/10.1186/1753-6561-5-S7-P178
Wang L (2014) Physiological and molecular responses to drought stress in rubber tree (Hevea brasiliensis Muell. Arg.). Plant Physiol Biochem 83:243–249. https://doi.org/10.1016/j.plaphy.2014.08.012
Lu Y, Zeng FJ, Li XY, Zhang B (2021) Physiological changes of three woody plants exposed to progressive salt stress. Photosynthetica 59(1):171–184. https://doi.org/10.32615/ps.2021.007
Abdolinejad R, Shekafandeh A (2022) Tetraploidy confers superior in vitro water-stress tolerance to the fig tree (Ficus carica) by reinforcing hormonal, physiological, and biochemical defensive systems. Front Plant Sci 12:796215. https://doi.org/10.3389/fpls.2021.796215
Rahman MM, Anamul-Haque M, Arafat-Islam-Nihad S, Mahmudul-Hasan-Akand M, Ruhul-Amin-Howlader M (2016) Morpho-physiological response of Acacia auriculiformis as influenced by seawater induced salinity stress. For Syst 25(3):e071. https://doi.org/10.5424/fs/2016253-09386
Tafreshi SAH, Aghaie P, Momayez HR, Hejaziyan SA (2021) Response of in vitro-regenerated Myrtus communis L. shoots to PEG-induced water stress. Biocatal Agric Biotechnol 34:102033. https://doi.org/10.1016/j.bcab.2021.102033
Sinhababu A, Banerjee A (2013) Comparative responses of four tree legumes plants to PEG-induced water stress at seedling stage. Plant Physiol 1(1):1–5. https://doi.org/10.1007/s11738-003-0022-3
Karimi S, Yadollahi A, Nazari-Moghadam R, Imani A, Arzani K (2012) In vitro screening of almond (Prunus dulcis (mill.)) genotypes for drought tolerance. J Biol Environ Sci 6(18):263–270
Rad PB, Roozban MR, Karimi S, Ghahremani R, Vahdati K (2021) Osmolyte accumulation and sodium compartmentation has a key role in salinity tolerance of Pistachios rootstocks. Agriculture 11(8):708. https://doi.org/10.3390/agriculture11080708
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29:23–39. https://doi.org/10.1677/jme.0.0290023
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3(3):71–85
Liu C, Xie T, Chen C, Luan A, Long J, Li C, Ding Y, He Y (2017) Genome-wide organization and expression profiling of the R2R3-MYB transcription factor family in pineapple (Ananas comosus). BMC Genomics 18:503. https://doi.org/10.1186/s12864-017-3896-y
Cao Y, Luo Q, Tian Y, Meng F (2017) Physiological and proteomic analyses of the drought stress response in Amygdalus mira (Koehne) Yüet Lu roots. BMC Plant Biol 17:53–69. https://doi.org/10.1186/s12870-017-1000-z
Robinet CV, Watkinson JI, Sioson AA, Ramakrishnan N, Heath LS, Grene R (2010) Differential expression of heat shock protein genes in preconditioning for photosynthetic acclimation in water-stressed loblolly pine. Plant Physiol Biochem 48:256–264. https://doi.org/10.1016/j.plaphy.2009.12.005
Kawai M, Pan L, Reed JC, Uchimiya H (1999) Evolutionally conserved plant homologue of the Bax inhibitor-1 (BI-1) gene capable of suppressing Bax-induced cell death in yeast. FEBS Lett 464(3):143–147. https://doi.org/10.1016/s0014-5793(99)01695-6
Jeong MJ, Choi BS, Bae DW, Shin SC, Park SU, Lim HS, Kim J, Kim JB, Cho BK, Bae H (2012) Differential expression of kenaf phenylalanine ammonia-lyase (PAL) ortholog during developmental stages and in response to abiotic stresses. Plant omics 5(4):392–399
Khakdana F, Alizadehb H, Ranjbarc M (2018) Molecular cloning, functional characterization and expression of a drought inducible phenylalanine ammonia-lyase gene (ObPAL) from Ocimum basilicum L. Plant Physiol Biochem 130:464–472. https://doi.org/10.1016/j.plaphy.2018.07.026
Behr M, Legay S, Hausman JF, Guerriero G (2015) Analysis of cell wall-related genes in organs of Medicago sativa L. under different abiotic stresses. Int J Mol Sci 16(7):16104–16124. https://doi.org/10.3390/ijms160716104
Mielke MS, Schaffer B, Li C (2010) Use of a SPAD meter to estimate chlorophyll content in Eugenia uniflora L. leaves as affected by contrasting light environments and soil flooding. Photosynthetica 48(3):332–338. https://doi.org/10.1007/s11099-010-0043-2
Premachandra GS, Saneoka H, Fujita K, Ogata S (1990) Cell membrane stability and leaf water relations as affected by nitrogen nutrition under water stress in maize. Soil Sci Plant Nutr 36(4):653–659. https://doi.org/10.1080/00380768.1990.10416802
Morgan JM (1984) Osmoregulation and water stress in higher plants. Ann Rev Plant Physiol 35:299–319. https://doi.org/10.1146/annurev.pp.35.060184.001503
Panse VG, Sukhatme PV (1978) Statistical methods for agricultural workers. Indian council of agricultural research, New Delhi, pp 87–89
Rahneshan Z, Nasibi F, Moghadam AA (2018) Effects of salinity stress on some growth physiological biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J Plant Interact 13(1):73–82. https://doi.org/10.1080/17429145.2018.1424355
Li Y, He N, Hou J, Xu L, Liu C, Zhang J, Wang Q, Zhang X, Wu X (2018) Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front Ecol Evol 6:64. https://doi.org/10.3389/fevo.2018.00064
Sneha C, Santhoshkumar AV, Sunil KM (2012) Effect of controlled irrigation on physiological and biometric characteristics in teak (Tectona grandis) seedlings. J Stress Physiol Biochem 8(3):196–202
Yue J, Fu Z, Zhang L, Zhang Z, Zhang J (2019) The positive effect of different 24-epibl pretreatments on salinity tolerance in Robinia pseudoacacia L. seedlings. Forests 10(1):4. https://doi.org/10.3390/f10010004
Zhang T, Zhao Y, Wang Y, Liu Z, Gao C (2018) Comprehensive analysis of MYB gene family and their expressions under abiotic stresses and hormone treatments in Tamarix hispida. Front Plant Sci 9:1303. https://doi.org/10.3389/fpls.2018.01303
Chen N, Feng J, Song B, Tang S, He J, Zhou Y, Shi S, Xu X (2019) De novo transcriptome sequencing and identification of genes related to salt and PEG stress in Tetraena mongolica Maxim. Trees-Struct Funct 33(6):1639–1656. https://doi.org/10.1007/s00468-019-01886-7
Zhou L, Yarra R, Jin L, Cao H (2020) Genome-wide identification and expression analysis of MYB gene family in oil palm (Elaeis guineensis Jacq.) under abiotic stress conditions. Environ Exp Bot 180:104245. https://doi.org/10.1016/j.envexpbot.2020.104245
Li D, Peng S, Chen S, Li Z, He Y, Ren B, Yang G (2021) Identification and characterization of 5 walnut MYB genes in response to drought stress involved in ABA signalling. Physiol Mol Biol Plants 27(6):1323–1335. https://doi.org/10.1007/s12298-021-01008-z
Liu J, Wang R, Liu W, Zhang H, Guo Y, Wen R (2018) Genome-wide characterization of heat-shock protein 70s from Chenopodium quinoa and expression analyses of Cqhsp70s in response to drought stress. Genes 9:35. https://doi.org/10.3390/genes9020035
Wang X, Tang C, Huang X, Li F, Chen X, Zhang G, Sun Y, Han D, Kang Z (2012) Wheat BAX inhibitor-1 contributes to wheat resistance to Puccinia striiformis. J Exp Bot 63(12):4571–4584. https://doi.org/10.1093/jxb/ers140
Lu PP, Zheng WJ, Wang CT, Shi WY, Fu JD, Chen M, Chen J, Zhou YB, Xi YJ, Xu ZS (2018) Wheat Bax Inhibitor-1 interacts with TaFKBP62 and mediates response to heat stress. BMC Plant Biol 18(1):259. https://doi.org/10.1186/s12870-018-1485-0
Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen HC, Zhang Y, Li X (2010) An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol 186:681–695. https://doi.org/10.1111/j.1469-8137.2010.03207.x
Ramiro DA, Passarin DMM, Barbosa MA, Santos F, Gomez SGP, Junior NSM, Lam E, Carrer H (2016) Expression of Arabidopsis Bax Inhibitor-1 in transgenic sugarcane confers drought tolerance. Plant Biotechnol J 14:1826–1837. https://doi.org/10.1111/pbi.12540
Watanabe N, Lam E (2006) Arabidopsis Bax inhibitor-1 functions as an attenuator of biotic and abiotic types of cell death. Plant J 45(6):884–894. https://doi.org/10.1111/j.1365-313X.2006.02654.x
Islam Z, Yun HK (2016) Identification and expression profiles of six transcripts encoding carboxylesterase protein in Vitis flexuosa infected with pathogens. Plant Pathol J 32(4):347–356. https://doi.org/10.5423/PPJ.OA.11.2015.0241
Li H, Han X, Qiu W, Xu D, Wang Y, Yu M, Hu X, Zhuo R (2019) Identification and expression analysis of the GDSL esterase/lipase family genes, and the characterization of SaGLIP8 in Sedum alfredii Hance under cadmium stress. Peer J 7:e6741. https://doi.org/10.7717/peerj.6741
Jahnen W, Hahlbrock K (1988) Differential regulation and tissue-specific distribution of enzymes of phenylpropanoid pathways in developing parsley seedlings. Planta 173(4):453–458. https://doi.org/10.1007/BF00958957
Guerriero G, Legay S, Hausman JF (2014) Alfalfa cellulose synthase gene expression under abiotic stress: a Hitchhiker’s guide to RT-qPCR normalization. PLoS ONE 9(8):e103808. https://doi.org/10.1371/journal.pone.0103808
Goncalves LP, Camargo RLB, Takita MA, Machado A, Filho WSS, Costa MGC (2019) Rootstock-induced molecular responses associated with drought tolerance in sweet orange as revealed by RNA-Seq. BMC Genomics 20:110. https://doi.org/10.1186/s12864-019-5481-z
Li Y, Cheng X, Fu Y, Wu Q, Guo Y, Peng J, Zhang W, He B (2019) A genome-wide analysis of the cellulose synthase-like (Csl) gene family in maize. Biol Plant 63:721–732. https://doi.org/10.32615/bp.2019.081
Zhao R, Cheng H, Wang Q, Lv L, Zhang Y, Song G, Zuo D (2022) Identification of the CesA subfamily and functional analysis of GhMCesA35 in Gossypium hirsutum L. Genes 13(2):292. https://doi.org/10.3390/genes13020292
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Research involving human and animal participants
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maisuria, H.J., Dhaduk, H.L., Kumar, S. et al. Physiological and gene expression responses involved in teak (Tectona grandis L.) seedlings exposed to osmotic and salt stressors. Mol Biol Rep 50, 4875–4886 (2023). https://doi.org/10.1007/s11033-023-08437-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08437-x



