Abstract
Background
Hypoxic pulmonary hypertension (HPH) is a complication of lung diseases with pulmonary vascular remodeling, although the underlying molecular mechanisms have not been fully elucidated. This study investigated the underlying molecular events by using a rat HPH model and primary pulmonary microvascular endothelial cells (PMVECs).
Methods and results
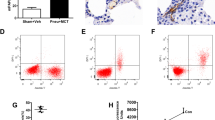
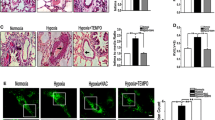
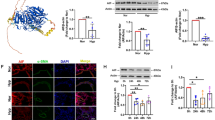
This study first established a rat HPH model and cultured PMVECs for transmission electron microscopic analysis and manipulation of 3-phosphoinositide-dependent protein kinase 1 (PDK1) or phosphatase and tensin homolog-induced kinase 1 (PINK1) expression in vitro. After that, the cell viability was assessed and the expression of different proteins was assayed using cell viability and western blot assays, respectively. Reactive oxygen species production, apoptosis, NLR family pyrin domain containing 3 (NLRP3) expression, and the levels of interleukin (IL)-1β, IL-6, and IL-8 were also assessed, while the interaction of PDK1 and PINK1 was determined using co-immunoprecipitation/western blot assays. Hypoxia induced mitophagy in the PMVECs and upregulated PINK1/Parkin expression, whereas knockdown of PINK1 expression under hypoxic conditions inhibited cell proliferation but induced endothelial cell apoptosis in vitro, decreased reactive oxygen species production and NLRP3 expression, and reduced the levels of inflammatory factors in PMVECs. However, hypoxia induced PDK1 expression, whereas knockdown of PDK1 downregulated PINK1 expression. Furthermore, treatment of the model rats with the PDK1 inhibitor dichloroacetate (DCA) was able to decrease PINK1 expression. In addition, the PDK1 and PINK1 proteins could interact with each other in the mitochondria of PMVECs to regulate the cell viability.
Conclusions
This study revealed that PDK1 induced PMVEC proliferation but inhibited their apoptosis to participate in pulmonary vascular remodeling, ultimately leading to HPH through regulation of PINK1-mediated mitophagy signaling. Therefore, PINK1 is a novel therapeutic target for the control of HPH.






Similar content being viewed by others
Data availability
The data that support the findings of this study are not publicly available due to none of the data types requiring uploading to a public repository but are available from the corresponding author upon reasonable request.
Abbreviations
- CCK-8:
-
cell counting kit-8
- DCA:
-
dichloroacetate
- HPH:
-
hypoxic pulmonary hypertension
- IL:
-
interleukin
- NLRP3:
-
NLR family pyrin domain containing 3
- PDK:
-
pyruvate dehydrogenase kinase
- PINK1:
-
phosphatase and tensin homolog-induced kinase 1
- PMVECs:
-
pulmonary microvascular endothelial cells
- ROS:
-
reactive oxygen species
- siRNA:
-
small interfering RNA
- VEGF:
-
vascular endothelial growth factor
- VEGFR:
-
vascular endothelial growth factor receptor
References
Howell K, Ooi H, Preston R, McLoughlin P (2004) Structural basis of hypoxic pulmonary hypertension: the modifying effect of chronic hypercapnia. Exp Physiol 89:66–72. https://doi.org/10.1113/expphysiol.2003.026765
Rowan SC, Keane MP, Gaine S, McLoughlin P (2016) Hypoxic pulmonary hypertension in chronic lung diseases: novel vasoconstrictor pathways. Lancet Respir Med 4:225–236. https://doi.org/10.1016/s2213-2600(15)00517-2
Nathan SD, Barbera JA, Gaine SP et al (2019) Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 53https//. https://doi.org/10.1183/13993003.01914-2018
McLaughlin VV, Archer SL, Badesch DB et al (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American college of cardiology foundation task force on expert consensus documents and the American heart association developed in collaboration with the American college of chest Physicians; American thoracic society, Inc.; and the pulmonary hypertension association. J Am Coll Cardiol 53:1573–1619. https://doi.org/10.1016/j.jacc.2009.01.004
Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF (2005) Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. Faseb j 19:1178–1180. https://doi.org/10.1096/fj.04-3261fje
Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM (2001) Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. Faseb j 15:427–438. https://doi.org/10.1096/fj.00-0343com
Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ (2005) Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96:442–450. https://doi.org/10.1161/01.RES.0000157672.70560.7b
Liao JK, Laufs U (2005) Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 45:89–118. https://doi.org/10.1146/annurev.pharmtox.45.120403.095748
Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF (2006) Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 291:L668–676. https://doi.org/10.1152/ajplung.00491.2005
Green DR, Galluzzi L, Kroemer G (2014) Cell biology. Metabolic control of cell death. Science 345:1250256. https://doi.org/10.1126/science.1250256
Xu W, Koeck T, Lara AR et al (2007) Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A 104:1342–1347. https://doi.org/10.1073/pnas.0605080104
Michelakis ED, Sutendra G, Dromparis P et al (2010) Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2:31ra34. https://doi.org/10.1126/scitranslmed.3000677
Stacpoole PW, Kurtz TL, Han Z, Langaee T (2008) Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev 60:1478–1487. https://doi.org/10.1016/j.addr.2008.02.014
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3:177–185. https://doi.org/10.1016/j.cmet.2006.02.002
McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED (2004) Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95:830–840. https://doi.org/10.1161/01.RES.0000145360.16770.9f
Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL (2002) Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105:244–250. https://doi.org/10.1161/hc0202.101974
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14. https://doi.org/10.1038/nrm3028
Dromparis P, Michelakis ED (2013) Mitochondria in vascular health and disease. Annu Rev Physiol 75:95–126. https://doi.org/10.1146/annurev-physiol-030212-183804
Vives-Bauza C, Zhou C, Huang Y et al (2010) PINK1-dependent recruitment of parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 107:378–383. https://doi.org/10.1073/pnas.0911187107
Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA (2012) Mitochondrial processing peptidase regulates PINK1 processing, import and parkin recruitment. EMBO Rep 13:378–385. https://doi.org/10.1038/embor.2012.14
Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ (2015) Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol 35:1166–1178. https://doi.org/10.1161/atvbaha.114.304865
Linqing L, Yuhan Q, Erfei L, Yong Q, Dong W, Chengchun T, Gaoliang Y, Bo L (2021) Hypoxia-induced PINK1/Parkin-mediated mitophagy promotes pulmonary vascular remodeling. Biochem Biophys Res Commun 534:568–575. https://doi.org/10.1016/j.bbrc.2020.11.040
Saraji A, Sydykov A, Schäfer K et al (2021) PINK1-mediated mitophagy contributes to pulmonary vascular remodeling in pulmonary hypertension. Am J Respir Cell Mol Biol 65:226–228. https://doi.org/10.1165/rcmb.2021-0082LE
Shimoda LA (2020) Cellular Pathways promoting pulmonary vascular remodeling by Hypoxia. Physiol (Bethesda) 35:222–233. https://doi.org/10.1152/physiol.00039.2019
Nozik-Grayck E, Stenmark KR (2007) Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol 618:101–112. https://doi.org/10.1007/978-0-387-75434-5_8
Warburg O, Posener K, Negelein E (1924) Über den Stoffwechsel der Carcinomzelle. Biochem Z 152:319–344
Dromparis P, Sutendra G, Michelakis ED (2010) The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl) 88:1003–1010. https://doi.org/10.1007/s00109-010-0670-x
Flores K, Siques P, Brito J, Arribas SM (2022) AMPK and the challenge of treating hypoxic pulmonary hypertension. Int J Mol Sci 23https//. https://doi.org/10.3390/ijms23116205
Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR (2015) The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308:L229–252. https://doi.org/10.1152/ajplung.00238.2014
Maxová H, Herget J, Vízek M (2012) Lung mast cells and hypoxic pulmonary hypertension. Physiol Res 61:1–11. https://doi.org/10.33549/physiolres.932221
Jernigan NL (2015) Smooth muscle acid-sensing ion channel 1: pathophysiological implication in hypoxic pulmonary hypertension. Exp Physiol 100:111–120. https://doi.org/10.1113/expphysiol.2014.081612
Herget J, Wilhelm J, Novotná J, Eckhardt A, Vytásek R, Mrázková L, Ostádal M (2000) A possible role of the oxidant tissue injury in the development of hypoxic pulmonary hypertension. Physiol Res 49:493–501
Ward JP, McMurtry IF (2009) Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 9:287–296. https://doi.org/10.1016/j.coph.2009.02.006
Stenmark KR, Tuder RM, El Kasmi KC (2015) Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119:1164–1172. https://doi.org/10.1152/japplphysiol.00283.2015
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462:245–253. https://doi.org/10.1016/j.abb.2007.03.034
Macleod KF (2020) Mitophagy and mitochondrial dysfunction in cancer. Annu Rev Cancer Biol 4:41–60. https://doi.org/10.1146/annurev-cancerbio-030419-033405
Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW (2014) Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal 20:474–494. https://doi.org/10.1089/ars.2013.5373
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J 26:1749–1760. https://doi.org/10.1038/sj.emboj.7601623
Villegas LR, Kluck D, Field C, Oberley-Deegan RE, Woods C, Yeager ME, El Kasmi KC, Savani RC, Bowler RP, Nozik-Grayck E (2013) Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal 18:1753–1764. https://doi.org/10.1089/ars.2012.4799
Soon E, Holmes AM, Treacy CM et al (2010) Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122:920–927. https://doi.org/10.1161/circulationaha.109.933762
Melser S, Chatelain EH, Lavie J et al (2013) Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab 17:719–730. https://doi.org/10.1016/j.cmet.2013.03.014
Liu Z, Chen X, Wang Y, Peng H, Wang Y, Jing Y, Zhang H (2014) PDK4 protein promotes tumorigenesis through activation of cAMP-response element-binding protein (CREB)-Ras homolog enriched in brain (RHEB)-mTORC1 signaling cascade. J Biol Chem 289:29739–29749. https://doi.org/10.1074/jbc.M114.584821
Acknowledgements
None.
Funding
This study was supported in part by grants from the National Natural Science Foundation of China (#81571839 and #82072102).
Author information
Authors and Affiliations
Contributions
BZ was mainly responsible for the design of the experiment and the writing of the article. JW was responsible for establishing the animal model, cell culture, the electron microscopy assay, and statistical analysis. YZ was responsible for the RT‑qPCR and western blot assays. YL was responsible for cell transfection, proliferation, and apoptosis investigations. ML was responsible for the CoIP assay. WN was responsible for reviewing and editing the manuscript. ZL was responsible for the design of the experiment. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Animal Use and Care Committee for Research and Education of The Fourth Military Medical University (Xi’an, China), and all experimental procedures followed the Guidelines of the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research. The study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Zhang, Y., Luo, Y. et al. PDK1 upregulates PINK1-mediated pulmonary endothelial cell mitophagy during hypoxia-induced pulmonary vascular remodeling. Mol Biol Rep 50, 5585–5596 (2023). https://doi.org/10.1007/s11033-023-08428-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08428-y