Abstract
Background
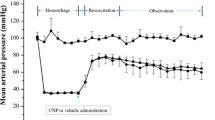
In this study, a comparison between centrally and systemically administered erythropoietin (EPO) was performed on nephroprotection during hemorrhagic shock (HS) in male rats.
Methods
Male rats were allocated into four experimental groups. (1) Sham; a guide cannula was inserted into the left lateral ventricle and other cannulas were placed into the left femoral artery and vein. (2) HS; stereotaxic surgery was done to insert a cannula in the left lateral ventricle and after a 7-day recovery; hemorrhagic shock and resuscitation were performed. (3) EPO-systemic; the procedure was the same as the HS group except that animals received 300 IU/kg erythropoietin into the femoral vein immediately before resuscitation. (4) EPO-central; animals was treated with erythropoietin (2 IU/rat) into the left lateral ventricle before resuscitation. Arterial oxygen saturation (SaO2) was measured during experiments. Urine and renal tissue samples were stored for ex-vivo indices assessments.
Results
Erythropoietin (systemically/centrally administered) significantly improved SaO2, renal functional and oxidative stress parameters and decreased renal inflammatory (TNF-α and IL-6) mRNA expression compared to the HS group. EPO-treated groups showed a decrease in active form of caspase-3 protein level and an increase in autophagy activity in comparison with the HS group.
Conclusion
Considering the fact that the effective dose of systemic EPO (300 IU/kg) was roughly 50 times higher than that of central administration (2 IU/rat), centrally administered EPO was accompanied by more advantageous consequences than systemic way. EPO is likely to act as a neuro-modulator or neuro-mediator in the central protection of organs including the kidneys.






Similar content being viewed by others
Data Availability
All data will be available by reasonable request.
References
Kholmukhamedov A, Czerny C, Hu J, Schwartz J, Zhong Z, Lemasters JJ (2014) Minocycline and doxycycline, but not tetracycline, mitigate liver and kidney injury after hemorrhagic shock/resuscitation. Shock 42(3):256–263
Ranjbaran M, Kadkhodaee M, Seifi B, Adelipour M, Azarian B (2017) Erythropoietin attenuates experimental haemorrhagic shock-induced renal damage through an iNOS- dependent mechanism in male Wistar rats. Injury 48(2):262–269
Kushimoto S, Kudo D, Kawazoe Y (2016) Acute traumatic coagulopathy and trauma-induced coagulopathy: an overview. J Intensive Care 5:1–7
Wang Y, Yan J, Xi L, Qian Z, Wang Z, Yang L (2012) Protective effect of crocetin on hemorrhagic shock-induced acute renal failure in rats. Shock (Augusta Ga) 38(1):63–67
Gilbert K, Rousseau G, Bouchard C, Dunberry-Poissant S, Baril F, Cardinal AM et al (2019) Caspase-(8/3) activation and organ inflammation in a rat model of resuscitated hemorrhagic shock: a role for uric acid. J Trauma Acute Care Surg 86(3):431–439
Wang L, Di L, Noguchi CT (2014) Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci 10(8):921–939
Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C et al (1995) Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A 92(9):3717–3720
Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V et al (1996) Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci 8(4):666–676
Ranjbaran M, Kadkhodaee M, Seifi B (2017) Renal tissue pro-inflammatory gene expression is reduced by erythropoietin in rats subjected to hemorrhagic shock. J Nephropathol 6(2):69–73
Yoo SJ, Cho B, Lee D, Son G, Lee YB, Soo Han H et al (2017) The erythropoietin-derived peptide MK-X and erythropoietin have neuroprotective effects against ischemic brain damage. Cell Death Dis 8(8):e3003
Hasselblatt M, Ehrenreich H, Siren AL (2006) The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol 18(2):132–138
Gu L, Xu H, Wang F, Xu G, Sinha D, Wang J et al (2014) Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Invest Ophthalmol Vis Sci 55(12):8208–8222
Seifi B, Kadkhodaee M, Ranjbaran M, Bakhshi E (2018) Nephroprotection through the Akt/eNOS pathway by centrally administered erythropoietin in a rat model of fixed-volume hemorrhage. Life Sci 193:180–185
Maiese K (2015) Erythropoietin and diabetes mellitus. World J Diabetes 6(14):1259
Zhong L, Zhang H, Ding Z-F, Li J, Lv J-W, Pan Z-J et al (2020) Erythropoietin-induced autophagy protects against spinal cord injury and improves neurological function via the extracellular-regulated protein kinase signaling pathway. Mol Neurobiol 57(10):3993–4006
Bendix I, Schulze C, Haefen Cv, Gellhaus A, Endesfelder S, Heumann R et al (2012) Erythropoietin modulates autophagy signaling in the developing rat brain in an in vivo model of oxygen-toxicity. Int J Mol Sci 13(10):12939–12951
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates. ed t
Ahmadi-Yazdi C, Williams B, Oakes S, Moore FD (2009) Jr. Attenuation of the effects of rat hemorrhagic shock with a reperfusion injury-inhibiting agent specific to mice. Shock 32(3):295–301
Seifi B, Kadkhodaee M, Bakhshi E, Ranjbaran M, Ahghari P, Rastegar T (2014) Enhancement of renal oxidative stress by injection of angiotensin II into the paraventricular nucleus in renal ischemia-reperfusion injury. Can J Physiol Pharmacol 92(9):752–757
Ali RJ, Al-Obaidi FH, Arif HS (2014) The role of urinary N-acetyl Beta-D-glucosaminidase in children with urological problems. Oman Med J 29(4):285–288
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106(1):207–212
Torres Filho IP, Torres LN, Pittman RN (2010) Early physiologic responses to hemorrhagic hypotension. Transl Res 155(2):78–88
Nandra KK, Collino M, Rogazzo M, Fantozzi R, Patel NS, Thiemermann C (2013) Pharmacological preconditioning with erythropoietin attenuates the organ injury and dysfunction induced in a rat model of hemorrhagic shock. Dis Model Mech 6(3):701–709
Teng Y, Zhang J, Zhang Z, Feng J (2018) The effect of chronic fluorosis on calcium ions and camkiialpha, and c-fos expression in the rat hippocampus. Biol Trace Elem Res 182(2):295–302
Devarajan P (2008) Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl 241:89–94
Skalova S (2005) The diagnostic role of urinary N-acetyl-beta-D-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Medica (Hradec Kralove) 48(2):75–80
Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72:219–246
Kumar M, Bhoi S (2015) Does erythropoietin reactivate bone marrow dysfunction in trauma hemorrhagic shock? Int J Crit Illn Inj Sci 5(4):230–231
Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG (2007) ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of akt in renal epithelial cells. Am J Physiol Renal Physiol 292:F440–F7
Li P, Shi M, Maique J, Shaffer J, Yan S, Moe OW et al (2020) Beclin 1/Bcl-2 complex-dependent autophagy activity modulates renal susceptibility to ischemia-reperfusion injury and mediates renoprotection by Klotho. Am J Physiol Renal Physiol 318(3):F772–F92
Posnere B, Tranf KA, Greeng DR, Xavierh RJ, Shawf SY, Clarked PG et al (2013) Autosis is a na, K-ATPase–regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia–ischemia. Proc Natl Acad Sci U S A 110(51):20364–20371
Chen W-T, Hung K-C, Wen M-S, Hsu P-Y, Chen T-H, Wang H-D et al (2013) Impaired leukocytes autophagy in chronic kidney disease patients. Cardiorenal Med 3(4):254–264
Xin W, Li Z, Xu Y, Yu Y, Zhou Q, Chen L et al (2016) Autophagy protects human podocytes from high glucose-induced injury by preventing insulin resistance. Metabolism 65(9):1307–1315
Andrianova NV, Jankauskas SS, Zorova LD, Pevzner IB, Popkov VA, Silachev DN et al (2018) Mechanisms of age-dependent loss of dietary restriction protective effects in acute kidney injury. Cells 7(10):178
Majeski AE, Dice JF (2004) Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol 36(12):2435–2444
Cao Z, Wang Y, Long Z, He G (2019) Interaction between autophagy and the NLRP3 inflammasome. Acta Biochim Biophys Sin 51(11):1087–1095
Bourhy L, Mazeraud A, Bozza FA, Turc G, Lledo P, Sharshar T (2022) Neuro-inflammatory response and brain-peripheral crosstalk in Sepsis and Stroke. Front Immunol 13:1–10
Ditting T, Hilgers KF, Stetter A, Linz P, Schonweiss C, Veelken R (2020) Renal sympathetic nerves modulate erythropoietin plasma levels after transient hemorrhage in rats. Am J Physiol Renal Physiol 318(3):F772–F92
Funding
This research was supported by a grant (no = 27721) from Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing interests declared.
Ethical approval
Experimental protocols and animal care methods in the experiments were approved by the Animal Experimental Committee at Tehran University of Medical Sciences (Project number: 27721-30-04-93, Approval ID: 27721).
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ranjbaran, M., Kadkhodaee, M., Adelipour, M. et al. A comparison between centrally and systemically administered erythropoietin on kidney protection in a model of fixed-volume hemorrhagic shock in male rats. Mol Biol Rep 50, 4781–4789 (2023). https://doi.org/10.1007/s11033-023-08412-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08412-6