Abstract
Background
Ferroptosis plays an important part in Acute lung injury (ALI) caused by sepsis. The six-transmembrane epithelial antigen of the prostate 1 (STEAP1) has potential effects on iron metabolism and inflammation but reports on its function in ferroptosis and sepsis-caused ALI are lacking. Here we explored the role of STEAP1 in sepsis-caused ALI and the possible mechanisms.
Methods and results
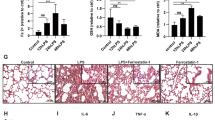
Lipopolysaccharide (LPS) was added to human pulmonary microvascular endothelial cells (HPMECs) to form the sepsis-caused ALI model in vitro. The Cecal ligation and puncture (CLP) experiment was performed on C57/B6J mice to form the sepsis-caused ALI model in vivo. The effect of STEAP1 on inflammation was investigated by PCR, ELISA, and Western blot for the inflammatory factors and adhesion molecular. The reactive oxygen species (ROS) levels were detected by immunofluorescence. The effect of STEAP1 on ferroptosis was investigated by detecting malondialdehyde (MDA) levels, glutathione (GSH) levels, Fe2+ levels, cell viability, and mitochondrial morphology. Our findings suggested that STEAP1 expression was increased in the sepsis-induced ALI models. Inhibition of STEAP1 decreased the inflammatory response and ROS production as well as MDA levels but increased the levels of Nrf2 and GSH. Meanwhile, inhibition of STEAP1 improved cell viability and restored mitochondrial morphology. Western Blot results showed that inhibition of STEAP1 could affect the SLC7A11/GPX4 axis.
Conclusion
Inhibition of STEAP1 may be valuable for pulmonary endothelial protection in lung injury caused by sepsis.





Similar content being viewed by others
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Singer M, Deutschman CS, Seymour CW et al (2016) The Third International Consensus Definitions for Sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810
Aird WC (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101(10):3765–3777
Gotts JE, Matthay MA (2016) Sepsis: pathophysiology and clinical management. BMJ (Clinical research ed) 353:i1585
Lum H, Roebuck KA (2001) Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280(4):C719–C741
Ware LB (2006) Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27(4):337–349
Nagase T, Ohga E, Sudo E et al (1996) Intercellular adhesion molecule-1 mediates acid aspiration-induced lung injury. Am J Respir Crit Care Med 154(2 Pt 1):504–510
Sun Y, Chen P, Zhai B et al (2020) The emerging role of ferroptosis in inflammation. Biomed pharmacotherapy = Biomedecine pharmacotherapie 127:110108
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Stockwell BR, Friedmann Angeli JP, Bayir H et al (2017) Ferroptosis: a regulated cell death Nexus linking metabolism, Redox Biology, and Disease. Cell 171(2):273–285
Liu P, Feng Y, Li H et al (2020) Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett 25:10
Zhou H, Li F, Niu JY et al (2019) Ferroptosis was involved in the oleic acid-induced acute lung injury in mice. Sheng Li Xue Bao 71(5):689–697
Wang YM, Gong FC, Qi X et al (2022) Mucin 1 Inhibits Ferroptosis and Sensitizes Vitamin E to Alleviate Sepsis-Induced Acute Lung Injury through GSK3β/Keap1-Nrf2-GPX4 Pathway. Oxidative medicine and cellular longevity ; 2022: 2405943
Finegold AA, Shatwell KP, Segal AW, Klausner RD, Dancis A (1996) Intramembrane bis-heme motif for transmembrane electron transport conserved in a yeast iron reductase and the human NADPH oxidase. J Biol Chem 271(49):31021–31024
Zhang X, Krause KH, Xenarios I, Soldati T, Boeckmann B (2013) Evolution of the ferric reductase domain (FRD) superfamily: modularity, functional diversification, and signature motifs. PLoS ONE 8(3):e58126
Ohgami RS, Campagna DR, Greer EL et al (2005) Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37(11):1264–1269
Hubert RS, Vivanco I, Chen E et al (1999) STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA 96(25):14523–14528
Ohgami RS, Campagna DR, McDonald A, Fleming MD (2006) The steap proteins are metalloreductases. Blood 108(4):1388–1394
Maia CJ, Socorro S, Schmitt F, Santos CR (2008) STEAP1 is over-expressed in breast cancer and down-regulated by 17beta-estradiol in MCF-7 cells and in the rat mammary gland. Endocrine ; 34(1–3): 108 – 16
Grunewald TG, Bach H, Cossarizza A, Matsumoto I (2012) The STEAP protein family: versatile oxidoreductases and targets for cancer immunotherapy with overlapping and distinct cellular functions. Biol Cell 104(11):641–657
Gomes IM, Maia CJ, Santos CR (2012) STEAP proteins: from structure to applications in cancer therapy. Mol cancer research: MCR 10(5):573–587
Grunewald TG, Diebold I, Esposito I et al (2012) STEAP1 is associated with the invasive and oxidative stress phenotype of ewing tumors. Mol cancer research: MCR 10(1):52–65
Liang Y, Xing X, Beamer MA et al (2017) Six-transmembrane epithelial antigens of the prostate comprise a novel inflammatory nexus in patients with pustular skin disorders. J Allergy Clin Immunol 139(4):1217–1227
Dejager L, Pinheiro I, Dejonckheere E, Libert C (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19(4):198–208
Oosterheert W, Gros P (2020) Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J Biol Chem 295(28):9502–9512
Kim K, Mitra S, Wu G et al (2016) Six-transmembrane Epithelial Antigen of prostate 1 (STEAP1) has a single b heme and is capable of reducing metal Ion Complexes and Oxygen. Biochemistry 55(48):6673–6684
Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black SM (2017) ROS Signaling in the pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv Exp Med Biol 967:105–137
Shaw P, Chattopadhyay A (2020) Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J Cell Physiol 235(4):3119–3130
Ji C, Kosman DJ (2015) Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J Neurochem 133(5):668–683
Yan HF, Zou T, Tuo QZ et al (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target therapy 6(1):49
Zink CF, Barker PB, Sawa A et al (2020) Association of missense mutation in FOLH1 with decreased NAAG levels and impaired Working Memory Circuitry and Cognition. Am J Psychiatry 177(12):1129–1139
Forcina GC, Dixon SJ (2019) GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 19(18):e1800311
Sato H, Tamba M, Ishii T, Bannai S (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274(17):11455–11458
Fujii J, Homma T, Kobayashi S (2020) Ferroptosis caused by cysteine insufficiency and oxidative insult. Free radical research ; 54(11–12): 969 – 80
Qu M, Zhang H, Chen Z et al (2021) The role of ferroptosis in Acute Respiratory Distress Syndrome. Front Med 8:651552
Funding
This study was supported by Chongqing Municipal Health Commission (project number cstc2021ycjh-bgzxm0129) and Chongqing Municipal Education Commission (project number lpjd202001).
Author information
Authors and Affiliations
Contributions
F.Z. and X.Z. designed the research; X.Z. performed the experiments, analyzed the data, and wrote the manuscript; C.L., Z.H., S.X., K.L. analyzed the data; Y.Y. and Y.H. performed the experiments. F.Z. revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
There are no competing interests declared by the authors.
Ethical approval
The NIH Guide for the Care and Use of Laboratory Animals was adhered to in all animal research and the protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No.2021 − 704).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, X., Liu, C., Huang, Z. et al. Inhibition of STEAP1 ameliorates inflammation and ferroptosis of acute lung injury caused by sepsis in LPS-induced human pulmonary microvascular endothelial cells. Mol Biol Rep 50, 5667–5674 (2023). https://doi.org/10.1007/s11033-023-08403-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08403-7