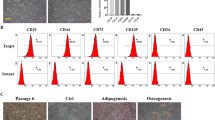
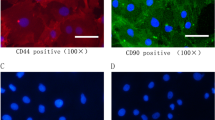
Abstract
Background
With advancing age of stem cells, dysregulation of various processes at the cellular level occurs, thereby decreasing their regeneration potential. One of the changes that occurs during the aging process is the accumulation of reactive oxygen species (ROS), which accelerates the processes of cellular senescence and cell death. The aim of this study is to evaluate two antioxidant compounds; Chromotrope 2B and Sulfasalazine, for their antioxidant effects on young and old rat bone marrow mesenchymal stem cells (MSCs).
Methods and results
Oxidative stress was induced in MSCs by 5 µM dexamethasone for 96 h and the cells were treated with Chromotrope 2B or Sulfasalazine, 50 µM each. The effects of antioxidant treatment following oxidative stress induction was evaluated by transcriptional profiling of genes involved in the oxidative stress and telomere maintenance. Expression levels of Cat, Gpx7, Sod1, Dhcr24, Idh1, and Txnrd2 were found to be increased in young MSCs (yMSCs) as a result of oxidative stress, while Duox2, Parp1, and Tert1 expression were found to be decreased as compared to the control. In old MSCs (oMSCs), the expressions of Dhcr24, Txnrd2, and Parp1 increased, while that of Duox2, Gpx7, Idh1, and Sod1 decreased following oxidative stress. In both MSC groups, Chromotrope 2B prompted decrease in the ROS generation before and after the induction of oxidative stress. In oMSCs, ROS content was significantly reduced in the Sulfasalazine treated group.
Conclusion
Our findings suggest that both Chromotrope 2B and Sulfasalazine possess the potential to reduce the ROS content in both age groups, though the latter was found to be more potent. These compounds can be used to precondition MSCs to enhance their regenerative potential for future cell-based therapeutics.




Similar content being viewed by others
References
Giri S, Machens HG, Bader A (2019) Therapeutic potential of endogenous stem cells and cellular factors for scar-free skin regeneration. Drug Discov Today 24(1):69–84
Caplan AI (2007) Adult mesenchymal stem cells for tissue Engineering Versus Regenerative Medicine. J Cell Physiol 213(2):341–347
Jiang XC, Xiang JJ, Wu HH, Zhang TY, Zhang DP, Xu QH, Huang XL, Kong XL, Sun JH, Hu YL, Li, Tabata Y, Shen YQ, Gao JQ (2019) Neural stem cells transfected with reactive oxygen species–responsive polyplexes for effective treatment of ischemic stroke. Adv Mater 31:1807591
Khorraminejad-Shirazi M, Mohammad Farahmandnia B, Kardeh A, Estedlal S, Kardeh A Monabati (2018) Aging and stem cell therapy: AMPK as an applicable pharmacological target for rejuvenation of aged stem cells and achieving higher efficacy in stem cell therapy. Hematol Oncol Stem Cell Ther 11(4):189–194
Li J, Zhang J, Chen Y, Kawazoe N, Chen G (2017) TEMPO-Conjugated gold nanoparticles for reactive oxygen species scavenging and regulation of stem cell differentiation. ACS Appl Mater Interfaces 9(41):35683–35692
Stefanatos R, Sanz A (2017) The role of mitochondrial ROS in the aging brain. Federation of European Biochemical Societies 592(2018):743–758
Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T (2004) Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431(7011):997–1002
Lau A, Kennedy BK, Kirkland JL, Tullius SG (2019) Mixing old and young: enhancing rejuvenation and accelerating aging. J Clin Invest 129(1):4–11
Zhao Y, Jia Z, Huang S, Wu Y, Liu L, Lin L, Wang D, He Q, Ruan D (2017) Age-related changes in nucleus pulposus mesenchymal stem cells: an in vitro study in rats. Stem Cells Int 6761572
Rossi DJ, Jamieson CH, Weissman IL (2008) Stems cells and the pathways to aging and cancer. Cell 132(4):681–696
Liao N, Shi Y, Zhang C, Zheng Y, Wang Y, Zhao B, Zeng Y, Liu X, Liu J (2019) Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res Ther 10(1):306
Tan SWS, Lee QY, Wong BSE, Cai Y, Baeg GH (2017) Redox homeostasis plays important roles in the maintenance of the Drosophila testis germline stem cells. Stem Cell Rep 9(1):342–354
Denu and Hematti (2016) Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxidative Medicine and Cellular Longevity 2016:1–2
Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH (2019) Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur J Cell Biol 98(5–8):151041
Moya A, Paquet J, Deschepper M, Larochette N, Oudina K, Denoeud C et al (2018) Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells 36:363–376
Hu C, Li L (2018) Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med 22(3):1428–1442
Valle-Prieto A, Conget PA (2010) Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19(12):1885–1893
Li M, Yu L, She T et al (2012) Astragaloside IV attenuates toll-like receptor 4 expression via NF-kappaB pathway under high glucose condition in mesenchymal stem cells. Eur J Pharmacol 696:203–209
Zhang LY, Xue HG, Chen JY et al (2016) Genistein induces adipogenic differentiation in human bone marrow mesenchymal stem cells and suppresses their osteogenic potential by upregulating PPARgamma. Exp Ther Med 11:1853–1858
Bhatti FU, Mehmood A, Latief N et al (2017) Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthritis Cartilage 25:321–331
Pourjafar M, Saidijam M, Mansouri K et al (2017) All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo. Cell Prolif 50:e12315
Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM (2020) Current knowledge and future perspectives on mesenchymal stem cell-derived Exosomes as a New Therapeutic Agent. Int J Mol Sci 21(3):727
Lin S, Zhao H, Xu C, Zhang P, Mei X, Jiang D (2023) Sulfasalazine-loaded nanoparticles for efficient inflammatory bowel disease therapy via ROS-scavenging strategy. Mater Design 225:111465
Ishii T, Mimura I, Nagaoka K et al (2022) Effect of M2-like macrophages of the injured-kidney cortex on kidney cancer progression. Cell Death Discov 8:480
Linares V, Alonso V, Domingo JL (2011) Oxidative stress as a mechanism underlying sulfasalazine-induced toxicity. Expert Opin Drug Saf 10(2):253–263
Kang C, Kim J, Ju S, Park S, Yoo J-W, Yoon I-S, Kim M-S, Jung Y (2022) Dapsone Azo-Linked with two Mesalazine Moieties is a “Me-Better” Alternative to Sulfasalazine. Pharmaceutics 14(3):684
Tourani S, Behvandi A (2022) Synthesis of MIL-101(cr)/Sulfasalazine (Cr-TA@SSZ) hybrid and its use as a novel adsorbent for adsorptive removal of organic pollutants from wastewaters. J Porous Mater 29:1441–1462
Kim JY, Cho HJ, Sir JJ, Kim BK, Hur J, Youn SW et al (2009) Sulfasalazine induces haem oxygenase-1 via ROS-dependent Nrf2 signalling, leading to control of neointimal hyperplasia. Cardiovasc Res 82(3):550–560
Joshi R, Kumar S, Unnikrishnan M, Mukherjee T (2005) Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radic Res 39(11):1163–1172
Haneef K, Naeem N, Khan I, Iqbal H, Kabir N, Jamall S, Zahid M, Salim A (2014) Conditioned medium enhances the fusion capability of rat bone marrow mesenchymal stem cells and cardiomyocytes. Mol Biol Rep 41:3099–3112
Khan I, Ali A, Akhter MA, Naeem N, Chotani MA, Iqbal H, Kabir N, Atiq M, Salim A (2017) Epac-Rap1-activated mesenchymal stem cells improve cardiac function in rat model of myocardial infarction. Cardiovasc Ther 35(2)
de Lange T (2002) Protection of mammalian telomeres. Oncogene 21(4):532–540
Costa TJ, Barros PR, Arce C, Santos JD, da Silva-Neto J, Egea G, Dantas AP, Tostes RC, Jiménez-Altayó F (2021) The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic Biol Med 162:615–635
Inoue T, Suzuki-Karasaki Y (2013) Mitochondrial superoxide mediates mitochondrial and endoplasmic reticulum dysfunctions in TRAIL-induced apoptosis in jurkat cells. Free Radic Biol Med 61:273–284
Bergaggio E, Piva R (2019 Apr 19) Wild-type IDH enzymes as actionable targets for Cancer Therapy. Cancers (Basel) 11(4):563
Nakamura H (2005) Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal 7(5–6):823–828
Tanaka C, Coling DE, Manohar S, Chen GD, Hu BH, Salvi R, Henderson D (2012) Expression pattern of oxidative stress and antioxidant defense-related genes in the aging Fischer 344/NHsd rat cochlea. Neurobiol Aging 33(8):1842e1–184214
Carmel-Harel O, Stearman R, Gasch AP, Botstein D, Brown PO, Storz G (2001) Role of thioredoxin reductase in the Yap1p-dependent response to oxidative stress in Saccharomyces cerevisiae. Mol Microbiol 39(3):595–605
Cox AG, Winterbourn CC, Hampton MB (2009) Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J 425(2):313–325
Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci (Qassim) 12(3):88–93
Dynek JN, Smith S (2004) Resolution of sister telomere association is required for progression through mitosis. Science 304:97–100
Smogorzewska A, de Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73:177–208
Tomás-Loba A, Flores I, Fernández-Marcos PJ, Cayuela ML, Maraver A, Tejera A, Borrás C, Matheu A, Klatt P, Flores JM, Viña J, Serrano M, Blasco MA (2008) Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135(4):609–622
Acknowledgements
This research article is dedicated to the memory of Dr. Siddiqua Jamall for her utmost support, guidance, and inspiration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Compliance with Ethical Standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iqbal, H., Naeem, N., Haneef, K. et al. Sulfasalazine and Chromotrope 2B reduce oxidative stress in murine bone marrow-derived mesenchymal stem cells. Mol Biol Rep 50, 4119–4131 (2023). https://doi.org/10.1007/s11033-023-08321-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08321-8