Abstract
Background
Hereditary hemochromatosis (HH) is characterized by iron overload that can cause multiple organ dysfunction primarily due to uncontrolled iron-mediated oxidative stress. Although HH leads to muscular weakness, disorder, and fatigue, the mechanism by which HH affects skeletal muscle physiology is largely unknown.
Methods
Using Hfe knockout mice (6–7 months old), a well-defined mouse model of HH, we examined iron status in the skeletal muscle, as well as other organs. As mitochondria are key organelle for muscular function, this study also explored how molecular markers for mitochondrial function and related systems are regulated in the HH skeletal muscle using western blots.
Results
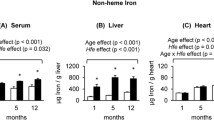
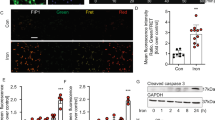
Although iron overload was evident at the systemic level, only mild iron overload was observed in the skeletal muscle of HH. Of note, mitochondrial electron transport chain complex I was upregulated in the HH skeletal muscle, which was accompanied by enhanced autophagy. However, these molecular changes were not associated with oxidative stress, suggesting altered mitochondrial metabolism in the muscle in response to iron overload.
Conclusions
These early adaptive responses may be important for supporting mitochondrial health before fully developing skeletal muscle dysfunction in HH. More studies are needed to determine the role of autophagy in the HH-related muscle mitochondrial dysfunction.






Similar content being viewed by others
References
Netz DJ et al (2011) Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol 8(1):125–132
Anderson CP et al (2012) Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 1823(9):1468–1483
Kassebaum NJ et al (2014) A systematic analysis of global anemia burden from 1990 to 2010. Blood 123(5):615–624
Sukumaran A et al (2017) Iron overload exacerbates age-associated cardiac hypertrophy in a mouse model of hemochromatosis. Sci Rep 7(1):5756
Mehta KJ, Farnaud SJ, Sharp PA (2019) Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol 25(5):521–538
Gordan R et al (2018) Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update. Heart Fail Rev 23(5):801–816
Winterbourn CC (1995) Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 82–83:969–974
D’Alessio F, Hentze MW, Muckenthaler MU (2012) The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol 57(5):1052–1060
Witte DL et al (1996) Hereditary hemochromatosis. Clin Chim Acta 245(2):139–200
Barton JC, Edwards CQ, Acton RT (2015) HFE gene: structure, function, mutations, and associated iron abnormalities. Gene 574(2):179–192
Niederau C et al (1996) Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 110(4):1107–1119
Allen MK (2010) Hereditary hemochromatosis: a literature review and case report. Physiother Can 62(3):276–284
Davidsen ES et al (2007) Reduced exercise capacity in genetic haemochromatosis. Eur J Cardiovasc Prev Rehabil 14(3):470–475
Reardon TF, Allen DG (2009) Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp Physiol 94(6):720–730
Alves FM et al (2022) Disruption of Hfe leads to skeletal muscle iron loading and reduction of hemoproteins involved in oxidative metabolism in a mouse model of hereditary hemochromatosis. Biochim Biophys Acta Gen Subj 1866(3):130082
Huang J et al (2010) Iron overload and diabetes risk: a shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 60(1):80–87
Kim J, Buckett PD, Wessling-Resnick M (2013) Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS ONE 8(5):e64944
Braymer JJ, Lill R (2017) Iron-sulfur cluster biogenesis and trafficking in mitochondria. J Biol Chem 292(31):12754–12763
Thomas HE et al (2018) Mitochondrial complex I activity is required for maximal autophagy. Cell Rep 24(9):2404–2417
Hou W et al (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12(8):1425–1428
Jahng JWS et al (2019) Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep 20(10):e47911
Fujimaki M et al (2019) Iron supply via NCOA4-mediated ferritin degradation maintains mitochondrial functions. Mol Cell Biol 39(14):e00010-19
Chao, X., et al., Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology, 2018. 155(3): p. 865–879 e12.
Agmon E et al (2018) Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci Rep 8(1):5155
Wang M et al (2015) Combined histological and hematological assessment of iron-induced organ damage in a gerbil model of iron overload. Am J Transl Res 7(2):385–392
Tamosauskaite J et al (2019) Hereditary hemochromatosis associations with frailty, sarcopenia and chronic pain: evidence from 200,975 older UK Biobank participants. J Gerontol A Biol Sci Med Sci 74(3):337–342
Munoz J, Ferrari N, Kuriakose P (2011) Iron-overload myopathy. Int J Hematol 94(6):503–504
Noetzli LJ et al (2008) Longitudinal analysis of heart and liver iron in thalassemia major. Blood 112(7):2973–2978
Levi S, Rovida E (2009) The role of iron in mitochondrial function. Biochim Biophys Acta 1790(7):629–636
Seo AY et al (2008) Mitochondrial iron accumulation with age and functional consequences. Aging Cell 7(5):706–716
Jang S-Y, Kang HT, Hwang ES (2012) Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem 287(23):19304–19314
Lin W, Kang UJ (2008) Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem 106(1):464–474
Roth JA et al (2010) Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J Neurochem 113(2):454–464
Ajoolabady A et al (2021) Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab 32(7):444–462
Rouault TA (2016) Mitochondrial iron overload: causes and consequences. Curr Opin Genet Dev 38:31–37
Kozhukhar N, Alexeyev MF (2019) Limited predictive value of TFAM in mitochondrial biogenesis. Mitochondrion 49:156–165
Acknowledgements
We would like to thank Ruiying Cheng and Lingxue Zeng for the initial animal setup.
Funding
This study was in part supported by the U.S. NIH/NINDS R21 NS121394.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Author V. Dhorajia declares that he has no conflict of interest. Author J. Kim declares that he has no conflict of interest. Author Y. Kim declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health, and the procedure was approved by University of Massachusetts Lowell Institutional Animal Care and Use Committee (IACUC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhorajia, V.V., Kim, J. & Kim, Y. Early adaptive responses in the skeletal muscle of young mice with hereditary hemochromatosis. Mol Biol Rep 50, 3179–3187 (2023). https://doi.org/10.1007/s11033-023-08264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08264-0