Abstract
Background
The effect of a novel brain-derived peptide, hypoxic-ischemic brain damage associated peptide (HIBDAP), on apoptosis after oxygen-glucose deprivation (OGD) in PC12 cells was investigated.
Methods
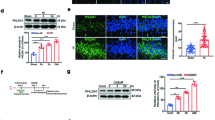
The HIBDAP sequence (HSQFIGYPITLFVEKER) was coupled with the carrier peptide of the transactivator of transcription (TAT) sequence (YGRKKRRQRRR). FITC-labelled TAT-HIBDAP was observed by fluorescence microscopy. After TAT-HIBDAP treatment and OGD treatment, the PC12 cell apoptosis rate was analysed using lactate dehydrogenase (LDH) leakage and Annexin V-fluorescein isothiocyanate (FITC) assays. Mitochondrial membrane potential (ΔΨm) was examined by fluorescence microscopy. Protein expression of apoptotic factors was examined by Western blotting.
Results
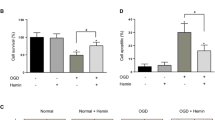
FITC-labelled TAT-HIBDAP entered the PC12 cell nucleus. Compared with the OGD group, TAT-HIBDAP at low concentrations (1 µM, 5 µM, 10 µM) significantly reduced the apoptosis rate of PC12 cells (except at 20 µM); 5 µM TAT-HIBDAP had the most obvious effect. There were remarkable increases in ΔΨm at different concentrations (1 µM, 5 µM, 10 µM, 20 µM) of TAT-HIBDAP pretreatment, and 5 µM TAT-HIBDAP also had the most obvious effect. TAT-HIBDAP reversed the increased ratio of Bax/Bcl-2 and activation of Caspase-3 induced by OGD.
Conclusion
TAT-HIBDAP is resistant to OGD-induced PC12 cell apoptosis by regulating the Bax/Bcl-2/Caspase-3 pathway, which may provide a novel therapeutic strategy for neonatal HIBD.





Similar content being viewed by others
Data availability
All supporting data are included within the main article.
References
Finder M, Boylan GB, Twomey D, Ahearne C, Murray DM, Hallberg B (2020) Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr 174(1):48–55
Natarajan G, Pappas A, Shankaran S (2016) Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin Perinatol 40(8):549–555
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20(1):122–128
Amatya R, Park T, Hwang S, Yang J, Lee Y, Cheong H et al (2020) Drug delivery strategies for enhancing the therapeutic efficacy of toxin-derived anti-diabetic peptides. Toxins (Basel) 12(5):313
Zhang X, Zhang Z, Xu X, Li Y, Li Y, Jian Y et al (2015) Bioinspired therapeutic dendrimers as efficient peptide drugs based on supramolecular interactions for Tumor Inhibition. Angew Chem Int Ed Engl 54(14):4289–4294
Cao SJ, Xu S, Wang HM, Ling Y, Dong J, Xia RD et al (2019) Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech 20(5):190
Hou X, Yuan Z, Wang X, Cheng R, Zhou X, Qiu J (2020) Peptidome analysis of cerebrospinal fluid in neonates with hypoxic-ischemic brain damage. Mol Brain 13(1):133
Zhu B, Yang J, Chen S, Zhang P, Shen L, Li X et al (2017) Oxymatrine on Hsp90a expression and apoptosis in a model of lung ischemia-reperfusion injury. Exp Ther Med 13(4):1381–1385
Rodriguez J, Li T, Xu Y, Sun Y, Zhu C (2021) Role of apoptosis-inducing factor in perinatal hypoxic-ischemic brain injury. Neural Regen Res 16(2):205–213
Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A (2008) A dual role of the NF-κB pathway in neonatal hypoxic-ischemic brain damage. Stroke 39(9):2578–2586
van der Kooij MA, Nijboer CH, Ohl F, Groenendaal F, Heijnen CJ, van Bel F et al (2010) NF-κB inhibition after neonatal cerebral hypoxia–ischemia improves long-term motor and cognitive outcome in rats. Neurobiol Dis 38(2):266–272
Zhou M, Xu W, Liao G, Bi X, Baudry M (2009) Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1α truncation. Exp Neurol 218(1):75–82
Donnini S, Solito R, Monti M, Balduini W, Carloni S, Cimino M et al (2009) Prevention of ischemic brain injury by treatment with the membrane penetrating apoptosis inhibitor, TAT-BH4. Cell Cycle 8(8):1271–1278
Sidhu RS, Tuor UI, Del Bigio MR (1997) Nuclear condensation and fragmentation following cerebral hypoxia-ischemia occurs more frequently in immature than older rats. Neurosci Lett 223(2):129–132
Zhang Y, Xu N, Ding Y, Doycheva DM, Zhang Y, Li Q et al (2019) Chemerin reverses neurological impairments and ameliorates neuronal apoptosis through ChemR23/CAMKK2/AMPK pathway in neonatal hypoxic-ischemic encephalopathy. Cell Death Dis 10(2):97
Banasiak KJ, Xia Y, Haddad GG (2000) Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol 62(2):215–249
Northington FJ, Graham EM, Martin LJ (2005) Apoptosis in perinatal hypoxic–ischemic brain injury: how important is it and should it be inhibited? Brain Res Brain Res Rev 50(2):244–257
Nijboer CH, Bonestroo HJ, Zijlstra J, Kavelaars A, Heijnen CJ (2013) Heijnen, mitochondrial JNK phosphorylation as a novel therapeutic target to inhibit neuroinflammation and apoptosis after neonatal ischemic brain damage. Neurobiol Dis 54:432–444
Lai Y, Chen Y, Watkins SC, Nathaniel PD, Guo F, Kochanek PM et al (2008) Identification of poly-ADP-ribosylated mitochondrial proteins after traumatic brain injury. J Neurochem 104(6):1700–1711
Iijima T (2006) Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res 55(3):234–243
Iijima T, Mishima T, Akagawa K, Iwao Y (2003) Mitochondrial hyperpolarization after transient oxygen-glucose deprivation and subsequent apoptosis in cultured rat hippocampal neurons. Brain Res 993(1–2):140–145
Rong Y, Distelhorst CW (2008) Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol 70:73–91
Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20(3):175–193
Taylor DL, Edwards AD, Mehmet H (1999) Oxidative metabolism, apoptosis and Perinatal Brain Injury. Brain Pathol 9(1):93–117
Abdel-Wahab A, Hassanin KMA, Mahmoud AA, Abdel-Badeea WIE, Abdel-Razik AH, Attia EZ et al (2021) Physiological roles of Red Carrot Methanolic extract and vitamin E to Abrogate Cadmium-Induced oxidative challenge and apoptosis in rat testes: involvement of the Bax/Bcl-2 ratio. Antioxid (Basel) 10(11):1653
Li Z, Xiao G, Wang H, He S, Zhu Y (2021) A preparation of Ginkgo biloba L. leaves extract inhibits the apoptosis of hippocampal neurons in post-stroke mice via regulating the expression of Bax/Bcl-2 and Caspase-3. J Ethnopharmacol 280:114481
Ku B, Liang C, Jung JU, Oh BH (2011) Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res 21(4):627–641
Van Opdenbosch N, Lamkanfi M (2019) Caspases in cell death, inflammation, and Disease. Immunity 50(6):1352–1364
Gill R, Soriano M, Blomgren K, Hagberg H, Wybrecht R, Miss MT et al (2002) Role of Caspase-3 activation in Cerebral Ischemia-Induced Neurodegeneration in Adult and neonatal brain. J Cereb Blood Flow Metab 22(4):420–430
Edwards AB, Anderton RS, Knuckey NW, Meloni BP (2018) Perinatal hypoxic-ischemic encephalopathy and neuroprotective peptide therapies: a case for cationic arginine-rich peptides (CARPs). Brain Sci 8(8):147
Meloni BP, Milani D, Edwards AB, Anderton RS, O’Hare Doig RL, Fitzgerald M et al (2015) Neuroprotective peptides fused to arginine-rich cell penetrating peptides: neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol Ther 153:36–54
Meloni BP, Mastaglia FL, Knuckey NW (2020) Cationic arginine-rich peptides (CARPs): a novel class of neuroprotective agents with a multimodal mechanism of action. Front Neurol 11:108
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81671500), 333 project of Jiangsu Province, and Nanjing Sanitation Engineering of Young Talents during the 13th Five-Year Plan Period (QRX17076).
Author information
Authors and Affiliations
Contributions
JQ conceptualized and designed the study, coordinated and supervised the experiments, provided research materials/reagents, reviewed and revised the manuscript. XH conducted the experiments and data interpretation. CJ and YH did the experiments, collected data, analyzed data and drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, C., Hu, Y., Hou, X. et al. Neuroprotective effect of a novel brain-derived peptide, HIBDAP, against oxygen-glucose deprivation through inhibition of apoptosis in PC12 cells. Mol Biol Rep 50, 3045–3051 (2023). https://doi.org/10.1007/s11033-023-08248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08248-0