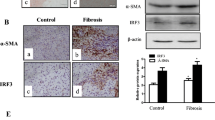
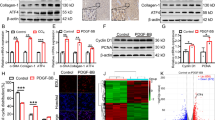
Abstract
Background
Tumor necrosis factor-α (TNFα) is a pleiotropic cytokine involved in nuclear factor kappa B (NF-κB) mediated cell survival as well as cell death. High serum TNFα levels correlate with liver fibrosis and enhance hepatic stellate cell (HSC) viability. However, the regulatory role of cellular inhibitor of apoptosis-1/2 (cIAP1/2) during TNFα induced NF-κB signaling in activated HSCs is largely unknown.
Method and Results
Activated HSCs were treated with cIAP1/2 inhbitiors i.e., SMAC mimetic BV6, and Birinapant in the presence of TNFα and macrophage conditioned media. TNFα cytokine increased cIAP2 expression and enhanced cell viability through the canonical NF-κB signaling in activated HSCs. cIAP2 inhibition via BV6 decreased the TNFα induced canonical NF-κB signaling, and reduced cell viability in activated HSCs. SMAC mimetic, Birinapant alone did not affect the cell viability but treatment of TNFα sensitized HSCs with Birinapant induced cell death. While BV6 mediated cIAP2 ablation was able to decrease the TNFα induced canonical NF-κB signaling, this effect was not observed with Birinapant treatment. Secreted TNFα from M1 polarized macrophages sensitized activated HSCs to BV6 or Birinapant mediated cell death. However, M2 polarized macrophage conditioned medium rescued the activated HSCs from BV6 mediated cytotoxicity.
Conclusion
In this study, we describe the regulatory role of cIAP2 in TNFα induced NF-κB signaling in activated HSCs. Targeting cIAP2 may be a promising approach for liver fibrosis treatment via modulating NF-κB signaling in activated HSCs.





Similar content being viewed by others
Data availability
The data acquired and analyzed in the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- TNFα :
-
Tumor necrosis factor-α
- cIAP1/2 :
-
Cellular inhibitor of apoptosis-1/2
- NF-κB :
-
Nuclear factor kappa B
- HSC :
-
Hepatic stellate cell
- αSMA :
-
α-Smooth muscle actin
- qHSC :
-
Quiescent HSC
- SMAC :
-
Second mitochondria-derived activator of caspase
- NASH :
-
Non-alcholic steatohepatitis
References
Yang YM, Seki E (2015) TNFα in liver fibrosis. Curr Pathobiol Rep 3(4):253–261
Osawa Y, Hoshi M, Yasuda I, Saibara T, Moriwaki H, Kozawa O (2013) Tumor necrosis factor-α promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS one 8(6):e65251
Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A et al (2001) Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34(6):1158–1163
Sudo K, Yamada Y, Moriwaki H, Saito K, Seishima M (2005) Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 29(5):236–244
Osawa Y, Kojika E, Hayashi Y, Kimura M, Nishikawa K, Yoshio S et al (2018) Tumor necrosis factor-α-mediated hepatocyte apoptosis stimulates fibrosis in the steatotic liver in mice. Hepatology Commun 2(4):407–420
Pradere J-P, Kluwe J, De Minicis S, Jiao J-J, Gwak G-Y, Dapito DH et al (2013) Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 58(4):1461–1473
Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ et al (2014) Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci 111(32):E3297–E3305
Nishio T, Hu R, Koyama Y, Liang S, Rosenthal SB, Yamamoto G et al (2019) Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol 71(3):573–585
Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A et al (2012) Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci 109(46):E3186–E3195
Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C et al (2012) Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci 109(24):9448–9453
Sharma S, Ghufran SM, Ghose S, Biswas S (2021) Cytoplasmic vacuolation with endoplasmic reticulum stress directs sorafenib induced non-apoptotic cell death in hepatic stellate cells. Sci Rep 11(1):3089
Sharma S, Kaufmann T, Biswas S (2017) Impact of inhibitor of apoptosis proteins on immune modulation and inflammation. Immunol Cell Biol 95(3):236–243
Fulda S, Vucic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 11(2):109–124
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131(4):669–681
Vince JE, Wong WW-L, Khan N, Feltham R, Chau D, Ahmed AU et al (2007) IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131(4):682–93
Ebert G, Allison C, Preston S, Cooney J, Toe JG, Stutz MD et al (2015) Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proc Natl Acad Sci 112(18):5803–5808
Oakley F, Teoh V, Ching ASG, Bataller R, Colmenero J, Jonsson JR et al (2009) Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 136(7):2334–44.e1
Oakley F, Meso M, Iredale JP, Green K, Marek CJ, Zhou X et al (2005) Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 128(1):108–120
De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA et al (2007) Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 132(5):1937–1946
Graber TE, Holcik M (2011) Distinct roles for the cellular inhibitors of apoptosis proteins 1 and 2. Cell Death Dis 2(3):e135
Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH (2009) cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res 69(5):1782–1791
Benetatos CA, Mitsuuchi Y, Burns JM, Neiman EM, Condon SM, Yu G et al (2014) Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-κB activation, and is active in patient-derived xenograft models. Mol Cancer Ther 13(4):867–879
van der Heide D, Weiskirchen R, Bansal R (2019) Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front Immunol 10:2852
Parola M, Pinzani M (2019) Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 65:37–55
Rudnick DA (2017) Antifibrotic therapies in liver disease: ready for primetime? Clinical liver disease 9(6):138–140
An P, Wei L-L, Zhao S, Sverdlov DY, Vaid KA, Miyamoto M et al (2020) Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat Commun 11(1):2362
Tsuchida T, Friedman SL (2017) Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14(7):397–411
Chang W-J, Song L-J, Yi T, Shen K-T, Wang H-S, Gao X-D et al (2015) Early activated hepatic stellate cell-derived molecules reverse acute hepatic injury. World J Gastroenterol 21(14):4184–4194
Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH et al (2013) Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 58(4):1461–1473
Conte D, Holcik M, Lefebvre CA, LaCasse E, Picketts DJ, Wright KE et al (2006) Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol 26(2):699–708
Moulin M, Anderton H, Voss AK, Thomas T, Wong WW-L, Bankovacki A et al (2012) IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J 31(7):1679–91
Condon SM, Mitsuuchi Y, Deng Y, LaPorte MG, Rippin SR, Haimowitz T et al (2014) Birinapant, a smac-mimetic with improved tolerability for the treatment of solid tumors and hematological malignancies. J Med Chem 57(9):3666–3677
Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E et al (2008) Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc Natl Acad Sci 105(33):11778–11783
Son G, Iimuro Y, Seki E, Hirano T, Kaneda Y, Fujimoto J (2007) Selective inactivation of NF-κB in the liver using NF-κB decoy suppresses CCl4-induced liver injury and fibrosis. Am J Physiol-Gastrointest Liver Physiol 293(3):G631–G9
Acknowledgements
The authors would would like appreciate laboratory members, Bornika Roy, Basundhra Das, Prachi Sharma, and Umesh Gautam for their helpful and constructive discussion. The authors are grateful to Dr. W. Selvamurthy and Prof B.C. Das for encouraging and supporting our research at the Amity Institute of Molecular Medicine and Stem Cell Research (AIMMSCR), Amity University, Uttar Pradesh.
Funding
This work was supported by fellowships and grant supports from the Council of Scientific & Industrial Research (CSIR-09/915(006)/2017-EMRI to S. Sharma), University Grants Commission (UGC Ref no-581/CSIR-UGC-NET June 2017 to S.M. Ghufran), Department of Biotechnology (DBT-102/IFD/SAN/3003/2017–2018 to S. Biswas) and Science and Engineering Research Board (SERB) (CRG/2018/003918 to S. Biswas and SERB- YSS/2015/000092 to S. Ghose) Indian Council of Medical Research (ICMR-2019–1306/SCR/ADHOC-BMS to S. Biswas), India.
Author information
Authors and Affiliations
Contributions
SMG and SS together designed the experiments, performed most of the biochemical and cellular experiments, acquired, analyzed, and interpreted the data with statistical validation. SG carried out ELISA, and critical revision of the manuscript. SB was involved in study concept and design, acquisition, analysis, and interpretation of data; drafting of the manuscript, critical revision of the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghufran, S.M., Sharma, S., Ghose, S. et al. Divergent effect of Birinapant, and BV6 SMAC mimetic on TNFα induced NF-κB signaling and cell viability in activated hepatic stellate cells. Mol Biol Rep 50, 2107–2117 (2023). https://doi.org/10.1007/s11033-022-08210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08210-6