Abstract
Background
Ureaplasma, a genus of the order Mycoplasmatales and commonly grouped with Mycoplasma as genital mycoplasma is one of the most common microbes isolated from women with infection/inflammation-associated preterm labor (PTL). Mycoplasma spp. produce sialidase that cleaves sialic acid from glycans of vaginal mucous membranes and facilitates adherence and invasion of the epithelium by pathobionts, and dysregulated immune response. However, whether Ureaplasma species can induce the production of sialidase is yet to be demonstrated. We examined U. parvum-infected vaginal epithelial cells (VECs) for the production of sialidase and pro-inflammatory cytokines.
Methods
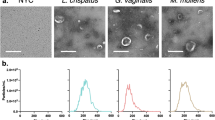
Immortalized VECs were cultured in appropriate media and treated with U. parvum in a concentration of 1 × 105 DNA copies/ml. After 24 h of treatment, cells and media were harvested. To confirm infection and cell uptake, immunocytochemistry for multi-banded antigen (MBA) was performed. Pro-inflammatory cytokine production and protein analysis for sialidase confirmed pro-labor pathways.
Results
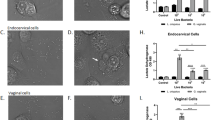
Infection of VECs was confirmed by the presence of intracellular MBA. Western blot analysis showed no significant increase in sialidase expression from U. parvum-treated VECs compared to uninfected cells. However, U. parvum infection induced 2-3-fold increased production of GM-CSF (p = 0.03), IL-6 (p = 0.01), and IL-8 (p = 0.01) in VECs compared to controls.
Conclusion
U. parvum infection of VECs induced inflammatory imbalance associated with vaginal dysbiosis but did not alter sialidase expression at the cellular level. These data suggest that U. parvum’s pathogenic effect could be propagated by locally produced pro-inflammatory cytokines and, unlike other genital mycoplasmas, may be independent of sialidase.



Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Jefferson KK (2012) The bacterial etiology of preterm birth. Adv Appl Microbiol 80:1–22. https://doi.org/10.1016/b978-0-12-394381-1.00001-5
Amabebe E, Anumba DOC (2018) The Vaginal Microenvironment: the physiologic role of Lactobacilli. Front Med 5(181). https://doi.org/10.3389/fmed.2018.00181
Amabebe E, Anumba DOC (2022) Mechanistic insights into Immune suppression and evasion in bacterial vaginosis. Curr Microbiol 79(3):84. https://doi.org/10.1007/s00284-022-02771-2
Rumyantseva T, Khayrullina G, Guschin A, Donders G (2019) Prevalence of Ureaplasma spp. and Mycoplasma hominis in healthy women and patients with flora alterations. Diagn Microbiol Infect Dis 93(3):227–231. https://doi.org/10.1016/j.diagmicrobio.2018.10.001
Miyoshi Y, Suga S, Sugimi S, Kurata N, Yamashita H, Yasuhi I (2022) Vaginal Ureaplasma urealyticum or Mycoplasma hominis and preterm delivery in women with threatened preterm labor. J Maternal-Fetal Neonatal Med 35(5):878–883. https://doi.org/10.1080/14767058.2020.1733517
Cox C, Saxena N, Watt AP, Gannon C, McKenna JP, Fairley DJ et al (2016) The common vaginal commensal bacterium Ureaplasma parvum is associated with chorioamnionitis in extreme preterm labor. J Matern Fetal Neonatal Med 29(22):3646–3651. https://doi.org/10.3109/14767058.2016.1140734
Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M et al (2006) Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 44(1):51–55. https://doi.org/10.1128/JCM.44.1.51-55.2006
Kacerovsky M, Kukla R, Bolehovska R, Bostik P, Matulova J, Mls J et al (2022) Prevalence and load of cervical Ureaplasma Species with respect to intra-amniotic complications in women with Preterm Prelabor rupture of membranes before 34 weeks. Front Pharmacol 13. https://doi.org/10.3389/fphar.2022.860498
Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD et al (2020) Intra-amniotic infection with Ureaplasma parvum causes Preterm Birth and neonatal mortality that are prevented by treatment with clarithromycin. mBio 11(3):e00797–e00720. https://doi.org/10.1128/mBio.00797-20
Viscardi RM (2010) Ureaplasma species: role in diseases of prematurity. Clin Perinatol 37(2):393–409. https://doi.org/10.1016/j.clp.2009.12.003
Sweeney EL, Dando SJ, Kallapur SG, Knox CL (2017) The human Ureaplasma species as causative agents of Chorioamnionitis. Clin Microbiol Rev 30(1):349–379. https://doi.org/10.1128/CMR.00091-16
Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ et al (2005) Relationship between antenatal inflammation and antenatal infection identified by detection of Microbial genes by polymerase chain reaction. Pediatr Res 57(4):570–577. https://doi.org/10.1203/01.PDR.0000155944.48195.97
Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS et al (2000) Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 183(5):1130–1137. https://doi.org/10.1067/mob.2000.109036
Šket T, Ramuta T, Starčič Erjavec M, Kreft ME (2021) The role of Innate Immune System in the human amniotic membrane and human amniotic fluid in Protection against intra-amniotic infections and inflammation. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.735324
Agarwal K, Lewis AL (2021) Vaginal sialoglycan foraging by Gardnerella vaginalis: mucus barriers as a meal for unwelcome guests? Glycobiology 31(6):667–680. https://doi.org/10.1093/glycob/cwab024
Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL (2013) Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem 288(17):12067–12079. https://doi.org/10.1074/jbc.M113.453654
Smith DG, Russell WC, Thirkell D (1994) Adherence of Ureaplasma urealyticum to human epithelial cells. Microbiol (Reading) 140(Pt 10):2893–2898. https://doi.org/10.1099/00221287-140-10-2893
Castro J, Machado D, Cerca N (2019) Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J 13(5):1306–1317. https://doi.org/10.1038/s41396-018-0337-0
Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, Musengamana V et al (2017) The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS ONE 12(2):e0172522. https://doi.org/10.1371/journal.pone.0172522
Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP (2001) Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect 77(6):402–408. https://doi.org/10.1136/sti.77.6.402
Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP et al (2005) Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106(5 Pt 1):1013–1023. https://doi.org/10.1097/01.AOG.0000183594.45524.d2
Swidsinski A, Mendling W, Loening-Baucke V, Swidsinski S, Dörffel Y, Scholze J et al (2008) An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 198(1):97e1–97e6. https://doi.org/10.1016/j.ajog.2007.06.039
Machado D, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N (2015) Bacterial vaginosis Biofilms: challenges to current therapies and emerging solutions. Front Microbiol 6:1528. https://doi.org/10.3389/fmicb.2015.01528
Basavaprabhu HN, Sonu KS, Prabha R (2020) Mechanistic insights into the action of probiotics against bacterial vaginosis and its mediated preterm birth: an overview. Microb Pathog 141:104029. https://doi.org/10.1016/j.micpath.2020.104029
Srinivasan U, Misra D, Marazita ML, Foxman B (2009) Vaginal and oral microbes, host genotype and preterm birth. Med Hypotheses 73(6):963–975. https://doi.org/10.1016/j.mehy.2009.06.017
Cauci S, Hitti J, Noonan C, Agnew K, Quadrifoglio F, Hillier SL et al (2002) Vaginal hydrolytic enzymes, immunoglobulin A against Gardnerella vaginalis toxin, and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol 187(4):877–881. https://doi.org/10.1067/mob.2002.127454
Cauci S, Thorsen P, Schendel DE, Bremmelgaard A, Quadrifoglio F, Guaschino S (2003) Determination of immunoglobulin A against Gardnerella vaginalis hemolysin, sialidase, and prolidase activities in vaginal fluid: implications for adverse pregnancy outcomes. J Clin Microbiol 41(1):435–438. https://doi.org/10.1128/jcm.41.1.435-438.2003
McGregor JA, French JI, Jones W, Milligan K, McKinney PJ, Patterson E et al (1994) Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol. ;170(4):1048-59; discussion 59–60. https://doi.org/10.1016/s0002-9378(94)70098-2
Robertson JA, Stemler ME, Stemke GW (1984) Immunoglobulin A protease activity of Ureaplasma urealyticum. J Clin Microbiol 19(2):255–258. https://doi.org/10.1128/jcm.19.2.255-258.1984
Paralanov V, Lu J, Duffy LB, Crabb DM, Shrivastava S, Methé BA et al (2012) Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvumstrains. BMC Microbiol 12(1):88. https://doi.org/10.1186/1471-2180-12-88
Kilian M, Brown MB, Brown TA, Freundt EA, Cassell GH (1984) Immunoglobulin A1 protease activity in strains of Ureaplasma urealyticum. Acta Pathologica Microbiologica Scandinavica Series B: Microbiology 92B(1–6):61–64. https://doi.org/10.1111/j.1699-0463.1984.tb02794.x
Ligon JV, Kenny GE (1991) Virulence of ureaplasmal urease for mice. Infect Immun 59(3):1170–1171. https://doi.org/10.1128/iai.59.3.1170-1171.1991
Glass JI, Lefkowitz EJ, Glass JS, Heiner CR, Chen EY, Cassell GH (2000) The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature 407(6805):757–762. https://doi.org/10.1038/35037619
Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA 2, Chesson R et al (2008) Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol 59(3):212–224. https://doi.org/10.1111/j.1600-0897.2007.00566.x
Tantengco OAG, Richardson L, Radnaa E, Kammala AK, Kechichian T, Ganguly E et al (2022) Cervix-on-a-chip for investigating ascending Ureaplasma parvum infection in pregnancy. Am J Obstet Gynecol 226(1):S12–S3. https://doi.org/10.1016/j.ajog.2021.11.068
Namba F, Hasegawa T, Nakayama M, Hamanaka T, Yamashita T, Nakahira K et al (2010) Placental features of Chorioamnionitis Colonized with Ureaplasma Species in Preterm Delivery. Pediatr Res 67(2):166–172. https://doi.org/10.1203/PDR.0b013e3181c6e58e
Flori F, Secciani F, Capone A, Paccagnini E, Caruso S, Ricci MG et al (2007) Menstrual cycle-related sialidase activity of the female cervical mucus is associated with exosome-like vesicles. Fertil Steril 88(4 Suppl):1212–1219. https://doi.org/10.1016/j.fertnstert.2007.01.209
Paolini L, Orizio F, Busatto S, Radeghieri A, Bresciani R, Bergese P et al (2017) Exosomes secreted by HeLa cells shuttle on their Surface the plasma Membrane-Associated Sialidase NEU3. Biochemistry 56(48):6401–6408. https://doi.org/10.1021/acs.biochem.7b00665
Cavanagh M, Amabebe E, Anumba DOC (2019) Differential Cytokine and Metabolite production by Cervicovaginal epithelial cells infected with Lactobacillus crispatus and Ureaplasma urealyticum. Anaerobe 102101. https://doi.org/10.1016/j.anaerobe.2019.102101
Uchida K, Nakahira K, Mimura K, Shimizu T, De Seta F, Wakimoto T et al (2013) Effects of Ureaplasma parvum lipoprotein multiple-banded antigen on pregnancy outcome in mice. J Reprod Immunol 100(2):118–127. https://doi.org/10.1016/j.jri.2013.10.001
Zheng X, Teng LJ, Watson HL, Glass JI, Blanchard A, Cassell GH (1995) Small repeating units within the Ureaplasma urealyticum MB antigen gene encode serovar specificity and are associated with antigen size variation. Infect Immun 63(3):891–898. https://doi.org/10.1128/iai.63.3.891-898.1995
Shimizu T, Kida Y, Kuwano K (2008) Ureaplasma parvum lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6. Microbiol (Reading) 154(Pt 5):1318–1325. https://doi.org/10.1099/mic.0.2007/016212-0
Triantafilou M, De Glanville B, Aboklaish AF, Spiller OB, Kotecha S, Triantafilou K (2013) Synergic activation of toll-like receptor (TLR) 2/6 and 9 in response to Ureaplasma parvum & urealyticum in human amniotic epithelial cells. PLoS ONE 8(4):e61199. https://doi.org/10.1371/journal.pone.0061199
Peltier MR, Freeman AJ, Mu HH, Cole BC (2007) Characterization of the macrophage-stimulating activity from Ureaplasma urealyticum. Am J Reprod Immunol 57(3):186–192. https://doi.org/10.1111/j.1600-0897.2006.00460.x
Sweeney EL, Kallapur SG, Meawad S, Gisslen T, Stephenson S-A, Jobe AH et al (2017) Ureaplasma Species multiple banded Antigen (MBA) variation is Associated with the severity of inflammation in vivo and in vitro in Human Placentae. Front Cell Infect Microbiol 7:123. https://doi.org/10.3389/fcimb.2017.00123
Pavlidis I, Spiller OB, Sammut Demarco G, MacPherson H, Howie SEM, Norman JE et al (2020) Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun 11(1):199. https://doi.org/10.1038/s41467-019-14089-y
Menon R, Behnia F, Polettini J, Richardson LS (2020) Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin Immunopathol 42(4):431–450. https://doi.org/10.1007/s00281-020-00808-x
Noda-Nicolau NM, Tantengco OAG, Polettini J, Silva MC, Bento GFC, Cursino GC et al (2022) Genital mycoplasmas and biomarkers of inflammation and their Association with spontaneous Preterm Birth and Preterm Prelabor rupture of membranes: a systematic review and Meta-analysis. Front Microbiol 13:859732. https://doi.org/10.3389/fmicb.2022.859732
Plummer EL, Vodstrcil LA, Bodiyabadu K, Murray GL, Doyle M, Latimer RL et al (2021) Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum Associated with specific genital symptoms and clinical signs in Nonpregnant Women? Clin Infect Dis 73(4):659–668. https://doi.org/10.1093/cid/ciab061
Cunha G, Bastos LB, Freitas SF, Cavalli RC, Quintana SM (2022) Genital mycoplasma infection and spontaneous preterm birth outcome: a prospective cohort study. BJOG 129(2):273–281. https://doi.org/10.1111/1471-0528.16949
Jonduo ME, Vallely LM, Wand H, Sweeney EL, Egli-Gany D, Kaldor J et al (2022) Adverse pregnancy and birth outcomes associated with Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum: a systematic review and meta-analysis. BMJ Open 12(8):e062990. https://doi.org/10.1136/bmjopen-2022-062990
Vornhagen J, Armistead B, Santana-Ufret V, Gendrin C, Merillat S, Coleman M et al (2018) Group B streptococcus exploits vaginal epithelial exfoliation for ascending infection. J Clin Invest 128(5):1985–1999. https://doi.org/10.1172/jci97043
Weed S, Armistead B, Coleman M, Liggit HD, Johnson B, Tsai J et al (2020) MicroRNA signature of epithelial-mesenchymal transition in Group B Streptococcal infection of the placental chorioamniotic membranes. J Infect Dis 222(10):1713–1722. https://doi.org/10.1093/infdis/jiaa280
Acknowledgements
We would like to thank the entire staff and students of The Menon’s lab at the Division of Basic Science and Translational Research, Department of Obstetrics and Gynaecology, University of Texas Medical Branch (UTMB), Texas, USA for their moral and technical support towards the success of Emmanuel’s visit and this project.
Funding
This work was funded by the Society for Reproductive Investigation International Training Grant 2020–2021 awarded to Dr Emmanuel Amabebe to visit Professor Ramkumar Menon’s laboratory at the Division of Basic Science and Translational Research, Department of Obstetrics and Gynecology, University of Texas Medical Branch (UTMB), Texas, USA.
Author information
Authors and Affiliations
Contributions
The study was conceived by EA, DA, LR, RM. The experiments and data analysis were conducted by EA, LR, GB, ER, TK and supervised by RM. The initial draft of the manuscript was written by EA, LR, GB, ER, and edited by all authors. All authors reviewed and approved the final draft of the manuscript for submission.
Corresponding authors
Ethics declarations
Conflicts of interest/Competing interests
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amabebe, E., Richardson, L.S., Bento, G.F.C. et al. Ureaplasma parvum infection induces inflammatory changes in vaginal epithelial cells independent of sialidase. Mol Biol Rep 50, 3035–3043 (2023). https://doi.org/10.1007/s11033-022-08183-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08183-6