Abstract
Background
Lacrimal adenoid cystic carcinoma (LACC) is the most common orbital malignant epithelial neoplasm. LACC with high-grade transformation (LACC-HGT) has higher rates of recurrence, metastasis, and mortality than LACC without HGT. This study investigated the effects of microRNA-29a-3p (miR-29a-3p) in the pathogenesis of LACC-HGT.
Methods
An Agilent human miRNA microarray was used to screen the differentially expressed miRNAs (DEMs) in LACC and LACC-HGT tumor tissues. Then, the primary cells obtained in previous studies were used to determine the effect of miR-29a-3p.
Results
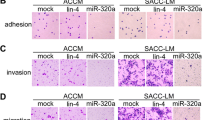
The expression of miR-29a-3p was abnormally lower in LACC-HGT than in LACC. miR-29a-3p can specifically target the 3ʹ UTR of Quaking mRNA and down-regulate Quaking expression, thereby inhibiting the proliferation, migration, and epithelial–mesenchymal transition of LACC cells.
Conclusions
This study illustrated that miR-29a-3p functions as a tumor suppressor by down-regulating the expression of Quaking to inhibit the tumorigenesis of LACC cells. This study may also reveal the pathogenesis of HGT in LACC cells and provide a reference for LACC-HGT targeted diagnosis.





Similar content being viewed by others
Data availability
The datasets used during the study available from the corresponding author on reasonable request.
Abbreviations
- DEMs:
-
The differentially-expressed miRNAs
- LACC:
-
Lacrimal gland adenoid cystic carcinoma
- LACC-HGT:
-
Lacrimal gland adenoid cystic carcinoma with high-grade transformation
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- miRNA:
-
Micro ribonucleic acid
- IHC:
-
Immunohistochemistry
- QKI:
-
KH domain containing RNA binding provided by HGNC
References
Nagao T (2013) “Dedifferentiation” and high-grade transformation in salivary gland carcinomas. Head Neck Pathol 7(Suppl 1):S37-47. https://doi.org/10.1007/s12105-013-0458-8
Panarelli JF, Zoumalan CI, Mukkamala K, Maher EA, Iacob C, Della RDA (2011) Dedifferentiated adenoid cystic carcinoma of the lacrimal gland. Ophthalmic Plast Reconstr Surg 27:e119-121. https://doi.org/10.1097/IOP.0b013e318201cb90
Seethala RR, Stenman G (2017) Update from the 4th edition of the world health organization classification of head and neck tumours: tumors of the Salivary Gland. Head Neck Pathol 11:55–67. https://doi.org/10.1007/s12105-017-0795-0
Tran TA, Jennings T, Carlson JA (2015) Dedifferentiated salivary hybrid carcinoma of the maxillary sinus with pagetoid spread to the overlying lining mucosa. Head Neck Pathol 9:293–299. https://doi.org/10.1007/s12105-014-0564-2
Cheuk W, Chan JK, Ngan RK (1999) Dedifferentiation in adenoid cystic carcinoma of salivary gland: an uncommon complication associated with an accelerated clinical course. Am J Surg Pathol 23:465–472. https://doi.org/10.1097/00000478-199904000-00012
Snyder ML, Paulino AF (1999) Hybrid carcinoma of the salivary gland: salivary duct adenocarcinoma adenoid cystic carcinoma. Histopathology 35:380–383. https://doi.org/10.1046/j.1365-2559.1999.00761.x
White VA (2012) Update on lacrimal gland neoplasms: Molecular pathology of interest. Saudi J Ophthalmol 26:133–135. https://doi.org/10.1016/j.sjopt.2012.02.012
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20:69–84. https://doi.org/10.1038/s41580-018-0080-4
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784. https://doi.org/10.1172/JCI20530
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. https://doi.org/10.1172/JCI39104
Ramesh V, Brabletz T, Ceppi P (2020) Targeting EMT in cancer with repurposed metabolic inhibitors. Trends Cancer 6:942–950. https://doi.org/10.1016/j.trecan.2020.06.005
Hellquist H, Skalova A, Barnes L, Cardesa A, Thompson LD, Triantafyllou A, Williams MD, Devaney KO, Gnepp DR, Bishop JA et al (2016) Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: a collective international review. Adv Ther 33:357–368. https://doi.org/10.1007/s12325-016-0298-5
Skalova A, Leivo I, Hellquist H, Agaimy A, Simpson RHW, Stenman G, Vander PV, Bishop JA, Franchi A, Hernandez-Prera JC et al (2021) High-grade transformation/dedifferentiation in salivary gland carcinomas: occurrence across subtypes and clinical significance. Adv Anat Pathol 28:107–118. https://doi.org/10.1097/PAP.0000000000000298
Pusztaszeri MP, Brochu V (2020) Metastatic adenoid cystic carcinoma with high-grade transformation (“dedifferentiation”) in pleural effusion and neck lymph node: a diagnostic challenge on cytology? Diagn Cytopathol 48:679–683. https://doi.org/10.1002/dc.24431
Ruggieri V, Russi S, Zoppoli P, La Rocca F, Angrisano T, Falco G, Calice G, Laurino S (2019) The role of MicroRNAs in the regulation of gastric cancer stem cells: a meta-analysis of the current status. J Clin Med. https://doi.org/10.3390/jcm8050639
Jin FE, Xie B, Xian HZ, Wang JH (2021) Knockdown of miR-125b-5p inhibits the proliferation and invasion of gastric carcinoma cells by targeting RYBP. Kaohsiung J Med Sci 37:863–871. https://doi.org/10.1002/kjm2.12425
Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK (2016) miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov 6:235–246. https://doi.org/10.1158/2159-8290.CD-15-0893
Wang M, Liao J, Tan C, Zhou H, Wang J, Wang K, Li Y, Wu W (2021) Integrated study of miR-215 promoting breast cancer cell apoptosis by targeting RAD54B. J Cell Mol Med 25:3327–3338. https://doi.org/10.1111/jcmm.16402
Yi B, Wang S, Wang X, Liu Z, Zhang C, Li M, Gao S, Wei S, Bae S, Stringer-Reasor E et al (2021) CRISPR interference and activation of the microRNA-3662-HBP1 axis control progression of triple-negative breast cancer. Oncogene. https://doi.org/10.1038/s41388-021-02089-6
He H, Wang N, Yi X, Tang C, Wang D (2017) Long non-coding RNA H19 regulates E2F1 expression by competitively sponging endogenous miR-29a-3p in clear cell renal cell carcinoma. Cell Biosci 7:65. https://doi.org/10.1186/s13578-017-0193-z
Kong Z, Wan X, Lu Y, Zhang Y, Huang Y, Xu Y, Liu Y, Zhao P, Xiang X, Li L et al (2020) Circular RNA circFOXO3 promotes prostate cancer progression through sponging miR-29a-3p. J Cell Mol Med 24:799–813. https://doi.org/10.1111/jcmm.14791
Li Y, Wang K, Wei Y, Yao Q, Zhang Q, Qu H, Zhu G (2017) lncRNA-MIAT regulates cell biological behaviors in gastric cancer through a mechanism involving the miR-29a-3p/HDAC4 axis. Oncol Rep 38:3465–3472. https://doi.org/10.3892/or.2017.6020
Shao NY, Wang DX, Wang Y, Li Y, Zhang ZQ, Jiang Q, Luo W, Cao C (2018) MicroRNA-29a-3p downregulation causes Gab1 upregulation to promote glioma cell proliferation. Cell Physiol Biochem 48:450–460. https://doi.org/10.1159/000491776
Zhao B, Song X, Guan H (2020) CircACAP2 promotes breast cancer proliferation and metastasis by targeting miR-29a/b-3p-COL5A1 axis. Life Sci 244:117179. https://doi.org/10.1016/j.lfs.2019.117179
Fagg W, Liu N, Fair J, Shiue L, Katzman S, Donohue J, Ares M (2017) QuakingAutogenous cross-regulation of mRNA processing and translation balances functions in splicing and translation. Genes Dev 31:1894–1909. https://doi.org/10.1101/gad.302059.117
Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ (2015) The RNA binding protein quaking regulates formation of circRNAs. Cell 160:1125–1134. https://doi.org/10.1016/j.cell.2015.02.014
Jiang M, Liu X, Zhang C, Zhu L, Wu HD, Dong L, Wang T, Lin T, He Y (2021) Bioinformatics identification of the candidate microRNAs and construction of a competing endogenous RNA regulatory network in lacrimal gland adenoid cystic carcinoma high-grade transformation. Oncol Lett 21:360. https://doi.org/10.3892/ol.2021.12621
Lin T, Zhu L, Zhou B, Xie L, Lv J, Dong L, He Y (2015) Establishment and characterization of a cell line from human adenoid cystic carcinoma of the lacrimal glands and a nude mouse transplantable model. Oncol Rep 33:2797–2806. https://doi.org/10.3892/or.2015.3925
Sato K, Ueda Y, Sakurai A, Ishikawa Y, Kaji S, Nojima T, Katsuda S (2006) Adenoid cystic carcinoma of the maxillary sinus with gradual histologic transformation to high-grade adenocarcinoma: a comparative report with dedifferentiated carcinoma. Virchows Arch 448:204–208. https://doi.org/10.1007/s00428-005-0054-8
Zhu Y, Zhu X, Xue X, Zhang Y, Hu C, Liu W, Lu H (2021) Exploration of high-grade transformation and postoperative radiotherapy on prognostic analysis for primary adenoid cystic carcinoma of the head and neck. Front Oncol 11:647172. https://doi.org/10.3389/fonc.2021.647172
Ge W, Goga A, He Y, Silva PN, Hirt CK, Herrmanns K, Guccini I, Godbersen S, Schwank G, Stoffel M (2021) miR-802 suppresses acinar-to-ductal reprogramming during early pancreatitis and pancreatic carcinogenesis. Gastroenterology. https://doi.org/10.1053/j.gastro.2021.09.029
Kalantzakos TJ, Sullivan TB, Gloria T, Canes D, Moinzadeh A, Rieger-Christ KM (2021) MiRNA-424-5p suppresses proliferation, migration, and invasion of clear cell renal cell carcinoma and attenuates expression of O-GlcNAc-transferase. Cancers (Basel). https://doi.org/10.3390/cancers13205160
Fan S, Chen WX, Lv XB, Tang QL, Sun LJ, Liu BD, Zhong JL, Lin ZY, Wang YY, Li QX et al (2015) miR-483-5p determines mitochondrial fission and cisplatin sensitivity in tongue squamous cell carcinoma by targeting FIS1. Cancer Lett 362:183–191. https://doi.org/10.1016/j.canlet.2015.03.045
Qiu H, Shen X, Chen B, Chen T, Feng G, Chen S, Feng D, Xu Q (2021) miR-30b-5p inhibits cancer progression and enhances cisplatin sensitivity in lung cancer through targeting LRP8. Apoptosis 26:261–276. https://doi.org/10.1007/s10495-021-01665-1
Wang Y, Feng J, Zang W, Du Y, Chen X, Sun Q, Dong Z, Zhao G (2015) MiR-499 enhances the cisplatin sensitivity of esophageal carcinoma cell lines by targeting DNA polymerase beta. Cell Physiol Biochem 36:1587–1596. https://doi.org/10.1159/000430321
Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu G, Zhao R, Huang H, Wang X, Qiao Y et al (2014) MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabine. Oncogene 33:378–386. https://doi.org/10.1038/onc.2012.575
Han J, Meng J, Chen S, Wang X, Yin S, Zhang Q, Liu H, Qin R, Li Z, Zhong W et al (2019) YY1 complex promotes quaking expression via super-enhancer binding during EMT of hepatocellular carcinoma. Cancer Res 79:1451–1464. https://doi.org/10.1158/0008-5472.CAN-18-2238
Chu L, Hu Y, Jiang YH, Xu C, Liu WC, Lu ZF (2019) Effects of RNA binding protein QKI on pancreatic cancer ductal epithelial cells and surrounding activation fibroblasts. J Cell Biochem. https://doi.org/10.1002/jcb.28435
Bian Y, Wang L, Lu H, Yang G, Zhang Z, Fu H, Lu X, Wei M, Sun J, Zhao Q et al (2012) Downregulation of tumor suppressor QKI in gastric cancer and its implication in cancer prognosis. Biochem Biophys Res Commun 422:187–193. https://doi.org/10.1016/j.bbrc.2012.04.138
Fu X, Feng Y (2015) QKI-5 suppresses cyclin D1 expression and proliferation of oral squamous cell carcinoma cells via MAPK signalling pathway. Int J Oral Maxillofac Surg 44:562–567. https://doi.org/10.1016/j.ijom.2014.10.001
Iwata N, Ishikawa T, Okazaki S, Mogushi K, Baba H, Ishiguro M, Kobayashi H, Tanaka H, Kawano T, Sugihara K, et al. (2017) Clinical significance of methylation and reduced expression of the quaking gene in colorectal cancer. Anticancer Res 37:489–498. https://doi.org/10.21873/anticanres.11341
Wang S, Tong X, Li C, Jin E, Su Z, Sun Z, Zhang W, Lei Z, Zhang HT (2021) Quaking 5 suppresses TGF-beta-induced EMT and cell invasion in lung adenocarcinoma. EMBO Rep 22:e52079. https://doi.org/10.15252/embr.202052079
Zhang RL, Yang JP, Peng LX, Zheng LS, Xie P, Wang MY, Cao Y, Zhang ZL, Zhou FJ, Qian CN et al (2016) RNA-binding protein QKI-5 inhibits the proliferation of clear cell renal cell carcinoma via post-transcriptional stabilization of RASA1 mRNA. Cell Cycle 15:3094–3104. https://doi.org/10.1080/15384101.2016.1235103
Yu F, Jin L, Yang G, Ji L, Wang F, Lu Z (2014) Post-transcriptional repression of FOXO1 by QKI results in low levels of FOXO1 expression in breast cancer cells. Oncol Rep 31:1459–1465. https://doi.org/10.3892/or.2013.2957
Acknowledgements
This study was performed at the Tianjin Medical University Eye Hospital.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 81570872), Tianjin Research Program of Application Foundation and Advanced Technology (Grant 15JCYBJC24900), Science and Technology Project of Tianjin Health Commission (Grant Numbers MS20025), Open Project of Tianjin Key Laboratory of Retinal Functions and Diseases (2021tjswmm001), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-037A).
Author information
Authors and Affiliations
Contributions
MX-J and F-X conceived the project. F-X and MX-J performed most of the experiment, collected the data. F-X wrote the manuscript. Q-T and JQ-L collected and analyzed the data. YJ-H, TT-L, LM-Z and JZ-Z made the surgeries for these patients. Pathological diagnosis was made by X-L. TT-L and CL-Z contributed to the interpretation of the data. TT-L and LJ-D made contributions to conception and design, and correction of the manuscript. TT-L provided the funding for the study. All authors have reviewed and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent to publish
All authors approved the manuscript for publication.
Ethical approval
Institutional of Human Research Ethics Committee of the Tianjin Medical University Eye Hospital approved this review [No. 2018KY(L)-02].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, F., Jiang, M., Tang, Q. et al. MiR-29a-3p inhibits high-grade transformation and epithelial–mesenchymal transition of lacrimal gland adenoid cystic carcinoma by targeting Quaking. Mol Biol Rep 50, 2305–2316 (2023). https://doi.org/10.1007/s11033-022-08150-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08150-1